FIG 3.
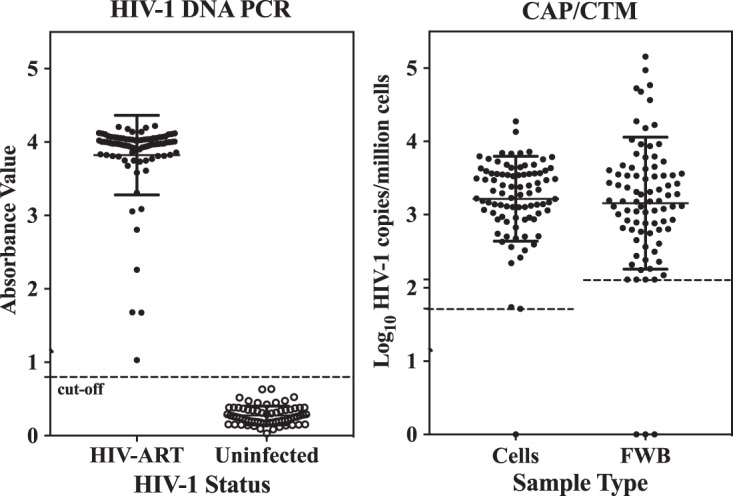
Detection of HIV-1 nucleic acid in frozen whole blood (FWB) and cell pellet samples of ART-treated HIV-1-infected individuals (chronic infection) with no detectable plasma HIV-1 RNA by the Roche Amplicor HIV-1 DNA PCR test, v1.5 (left), and the Roche CAP/CTM assay (right). The dashed lines represent the assay lower-limit cutoff. The CAP/CTM assay results were adjusted for cell input. Samples in which CAH was detected but not quantified by the Roche CAP/CTM assay are represented at the lower-limit cutoff. Roche CAP/CTM assay results are not shown on the right for uninfected individuals, as all test results were target not detected. Assignment of a value of 1 to results of target not detected provides a log10 value of 0, placing all results of target not detected on the x axis. The LOQ for cell pellets was 1.72 log10 copies/million cells, and that for FWB was 2.1 log10 copies/million cells.
