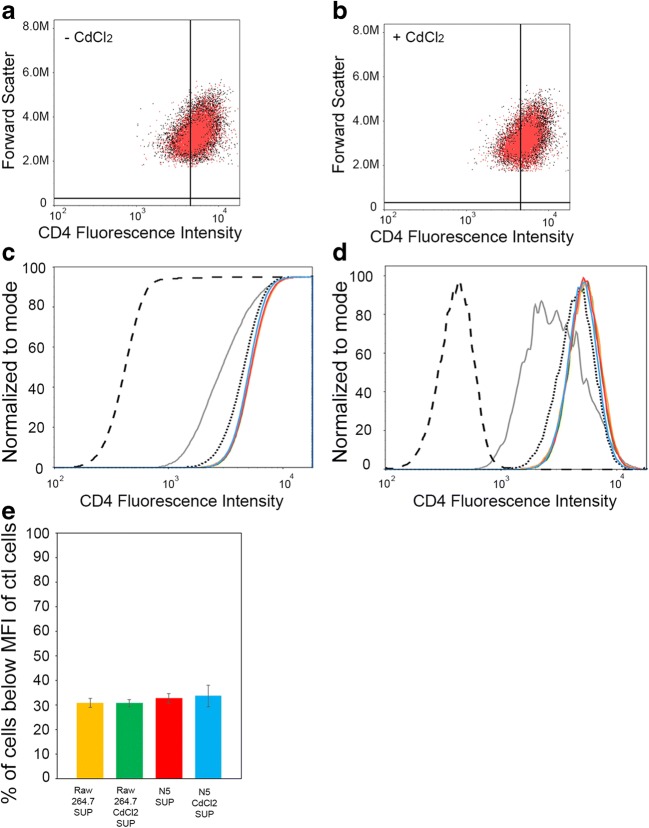
Fig. 5. Nef transfer to CEM-T4 requires direct cell-to-cell contact between donor and acceptor cells.
CEM-T4 were mixed in a 1 to 3 ratio with Raw 264.7 cells and incubated for 24 h with the supernatant from Raw 264.7 (± CdCl2) or the supernatant of N5 (± CdCl2). The mixed population of cells were analyzed by flow cytometry for cell surface CD4 staining. Representative 2-D scatter plots of the CD4 fluorescence intensity (log scale) in CEM-T4 mixed with supernatant from N5 (red) vs supernatant from Raw 264.7 (black) not-treated (a) or treated with CdCl2 (b). Representative CDF (c) and histogram (d) views are plotted. Black dashed line is CD4 negative control; black dotted line is CD4 positive control; gray is Nef expression in CEM-T4; blue is CEM-T4 cells co-cultured with the supernatant of CdCl2 treated N5 cells; red is CEM-T4 cells co-cultured with the supernatant of untreated N5 cells; yellow is CEMT4 cells co-cultured with the supernatant of untreated RAW 264.7 cells; green is CEM-T4 cells co-cultured with the supernatant of CdCl2 treated RAW 264.7 cells. (e) Graphical representation of (c-d) plotting the percent of CEM-T4 cells with a Mean Fluorescence Intensity (MFI) below that of positive control cells (CD4 labeled CEM-T4) from 3 independent flow cytometry experiments for all 4 culture conditions. No statistically significant changes of the MFI of CEM-T4 cells were observed under any conditions