Table 1. Optimization of the reaction conditions. 1a (0.1 mmol), DBAD (0.15 mmol), CeCl3·7H2O (10 mol%), CH3CN (0.1 M) at 25 °C, 455 nm LED for 24 h.
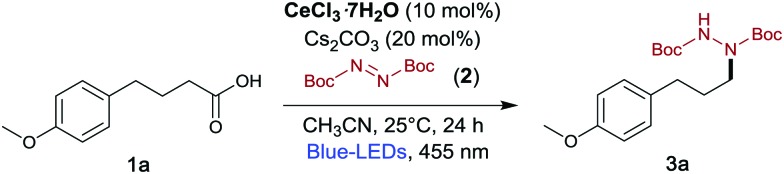
| ||
| Entry | Deviation from standard conditions | 3a a (%) |
| 1 | None | 90 (80) b |
| 2 | CeCl3 instead of CeCl3·7H2O | 80 |
| 3 | Ce(OTf)3 instead of CeCl3·7H2O | 66 |
| 4 | Ce(OTf)4 instead of CeCl3·7H2O | 63 |
| 5 | K2CO3 instead of Cs2CO3 | 66 |
| 6 | Na2CO3 instead of Cs2CO3 | 55 |
| 7 | Li2CO3 instead of Cs2CO3 | 25 |
| 8 | NaHCO3 instead of Cs2CO3 | 23 |
| 9 | Without base | 20 |
| 10 | DCM instead of CH3CN | 85 |
| 11 | DCE instead of CH3CN | 86 |
| 12 | CHCl3 instead of CH3CN | 66 |
| 13 | DMSO instead of CH3CN | 61 |
| 14 | Without light | 0 |
| 15 | Without CeCl3·7H2O | 0 |
aNMR yields using benzoyl benzoate as internal standard.
bIsolated yield.
