Fig. 2. Characterization of p95HER2-TCB.
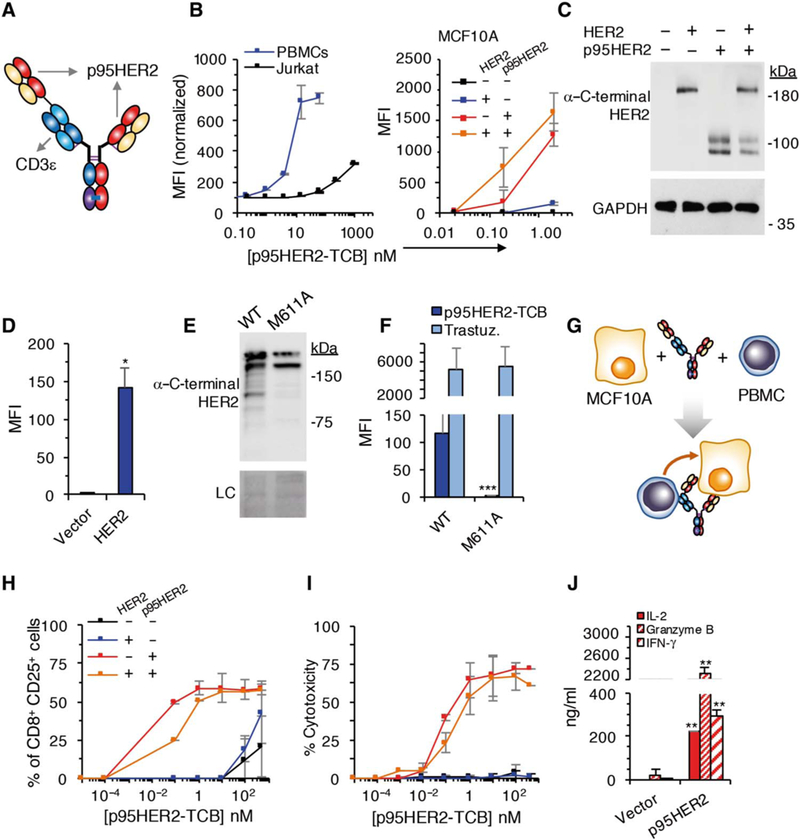
(A) Schematic showing the structure of p395HER2-TCB. (B) Median fluorescence intensity (MFI) of binding of p95HER2-TCB to human Jurkat cells and PBMCs (left) or MCF10A cells expressing empty vector or the same vector encoding HER2 and/or p95HER2 (right) (n = 3 expressed as means ± SD, measured by flow cytometry). (C) The same MCF10A cells as in (B) (right) expressing the indicated constructs were analyzed by Western blotting with antibodies directed against the C terminus of HER2. (D)MFI ofbindingofp95HER2-TCB to control MCF10A cells or the same cells over-expressing full-length HER2 (n = 3 expressed as means ± SD). (E) Lysates from MCF10A cells transfected with wild-type (WT) HER2 or HER2 bearing an M611A mutation were analyzed by Western blotting with anti-HER2 antibodies. Ponceau staining is shown as loading control (LC). (F) The same cells as in (E) were analyzed by flow cytometry with the indicated antibodies (n = 3 expressed as means ± SD). (G) Schematic drawing illustrating the coculture experiments to monitor the activity of p95HER2-TCB. (H and I) MCF10A cells transfected with the indicated vectors were incubated with PBMCs and different concentrations of p95HER2-TCB for 48 hours. Then, the expression of the activation marker CD25 on CD8+ cells was assessed by flow cytometry (H), and cyto-toxicity was determined by measuring lactate dehydrogenase (LDH) release (I) (n = 3 expressed as means ± SD). (J) MCF10A p95HER2 cells were incubated with PBMCs and 10 nM p95HER2-TCB, and the production of the indicated factors was determined by enzyme-linked immunosorbent assay (n = 3 expressed as means ± SD). (D, F, and J) *P < 0.05, **P < 0.01, ***P < 0.001, two-tailed t test.
