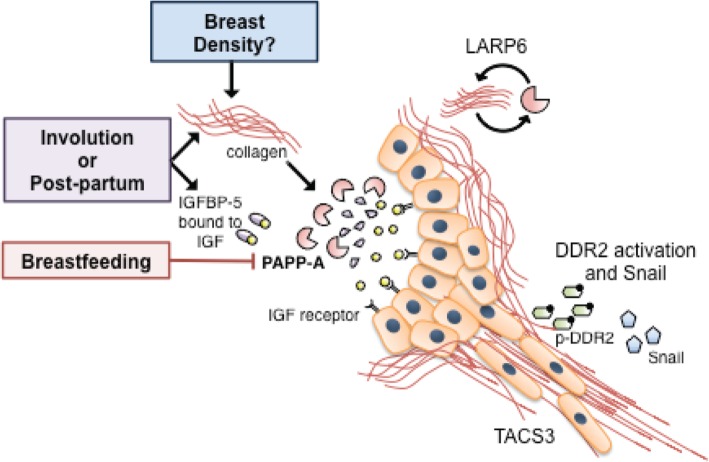
Fig. 7.
Model of PAPP-A-driven PABC. Schematic of our current understanding of PAPP-A-driven PABC, where red boxes indicate factors that have been established to activate the pathway, blue boxes indicate factors inhibiting the pathway, and green boxes factors that are hypothesized to affect the pathway. In this model, elevated content in collagen is necessary for PAPP-A to cleave IGFBP-5 in the mammary gland. The elevated level of collagen can be provided through distinct avenues including involution and, as demonstrated in the current study, post-partum environment. We postulate that high breast density may contribute the activity of PAPP-A. Conversely, extended breastfeeding appears to inhibit PAPP-A. Upon activation of PAPP-A and cleavage of IGFBP-5, free IGFs are released and able to bind and activate the IGF receptor, which results in increased IGF signaling, increased collagen deposition, and extended involution, in the case of an involuting mammary gland. We show that LARP6 is activated following PAPP-A overexpression and as a chaperone of the mRNA of collagen, LARP6 contributes to the elevation in collagen deposition in PAPP-A-driven mammary tumors. In addition, the collagen receptor DDR2 is activated, which leads to the DDR2/Snail axis of metastasis reported by others. In a mechanism that remains unclear, DDR2 promotes the formation of TACS3 collagen, which facilitates metastasis