FIGURE 6.
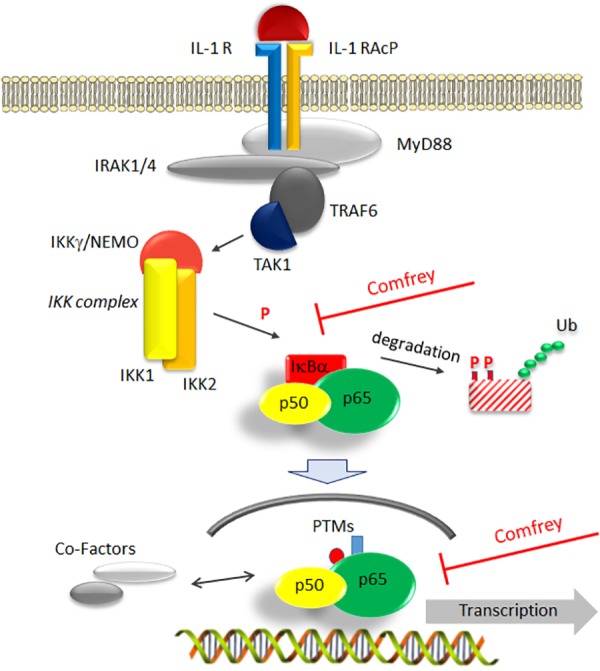
Model of comfrey’s mode of action by interference with the NF-κB signaling pathway. IL-1β binding to its receptor (IL-1 R) recruits an accessory protein (IL-1 RAcP) to activate a downstream signaling cascade involving MyD88, IRAK, and TRAF6. Of central importance is the activation of the IKK complex consisting of IKK-1, -2, and NEMO/IKKγ, which triggers the degradation of the main NF-κB inhibitor IκBα through phosphorylation (P) and ubiquitination (Ub). Liberation from IκBα enables NF-κB (depicted as a p50/p65 heterodimer) to translocate to the nucleus. Here, NF-κB can be modified further (post-translational modifications, PTMs) and interact with a variety of cofactors such as histone modifying proteins that modulate its transactivation potential. Based on our studies, the hydroalcoholic extract of comfrey root, as well as the mucilage-depleted fraction thereof, interfere at two points with this signaling cascade as indicated: first, IκBα phosphorylation and its subsequent degradation are delayed and second, transactivation of nuclear p65 becomes impaired. Together, this results in the alteration of pro-inflammatory gene expression, thus attenuating the further development of a pro-inflammatory scenario.
