Scheme 2.
Diene 1,4-Bromoazidation and Application to the Synthesis of Aza-Sugars
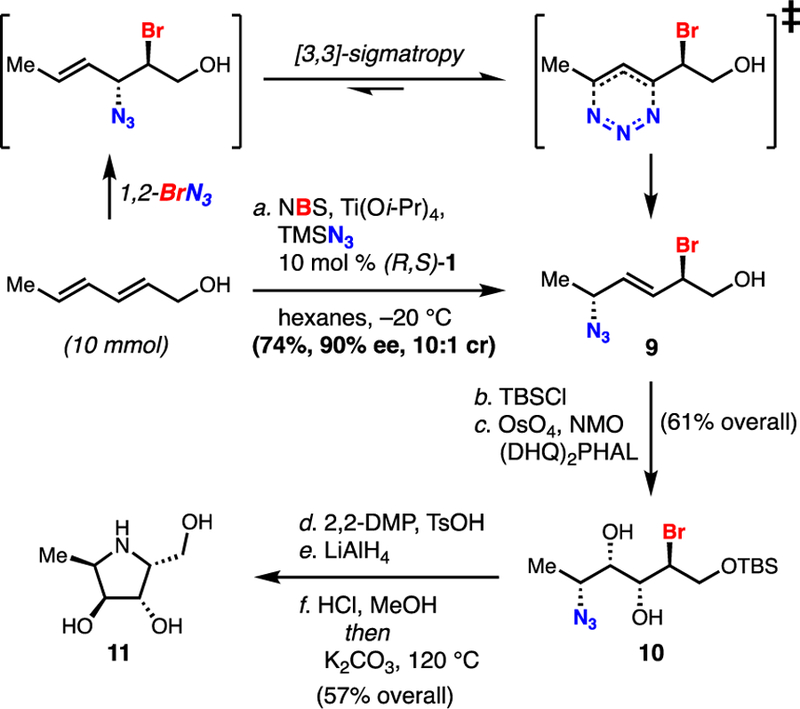
Conditions: a. 10.0 mmol allylic alcohol, 1.3 equiv of NBS, 1.2 equiv Ti(Oi-Pr)4, 1.1 equiv TMSN3, 10 mol % (R,S)-1, hexanes, –20 °C, 74%, 90% ee; b. TBSCl (2 equiv), Et3N (1.5 equiv), DMAP (0.5 equiv), DCM, RT, 90%; c. OsO4 (2 mol %), (DHQ)2PHAL (6 mol %), NMO (2 equiv), acetone/pH 7 buffer, 0 °C to RT, 68%; d. TsOH·H2O (10 mol %), 2,2-dimethoxypropane/acetone, RT, 92%; e. LiAlH4 (3 equiv), THF, –78 °C to RT, 72%; f. 6N HCl, MeOH, H2O, then K2CO3 (14 equiv), 120 °C (µw), 6 h, 87%.
