Figure 2.
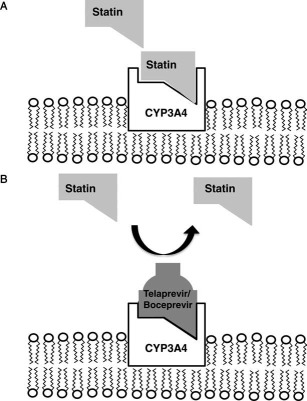
Mechanism for the competitive inhibition of CYP3A enzyme activity. (A) Many commonly used drugs such as atorvastatin have a strong binding affinity for CYP3A4, which is located in the endoplasmic reticulum of hepatocytes. In the steady state, the drug is absorbed via the gastrointestinal tract, metabolized by CYP3A4 in the liver, and eliminated from the body. (B) When a drug with a similar or greater binding affinity to CYP3A4 (e.g., telaprevir or boceprevir) is coadministered with atorvastatin, it can displace atorvastatin from CYP3A4 and lead to greater local and systemic bioavailability of the parent compound. Increased bioavailability of the drug can lead to a greater pharmacodynamic effect (e.g., hypolipidemia) as well as an increased incidence or severity of adverse events (e.g., myopathy) because of reduced drug metabolism.
