Figure 3.
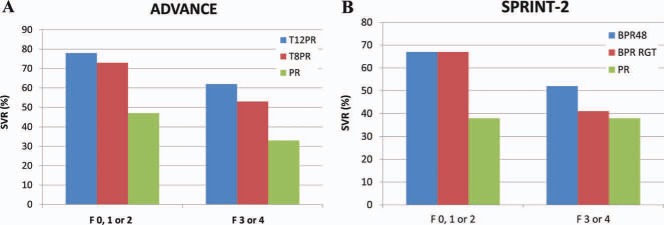
SVR rates for patients with no, mild, or portal fibrosis (Metavir F0, F1, or F2) or bridging fibrosis and cirrhosis (Metavir F3 or F4) in (A) the ADVANCE trial2 and (B) the SPRINT‐2 trial.3 Abbreviations: BPR, boceprevir and pegylated interferon/ribavirin; BPR48, boceprevir and pegylated interferon/ribavirin for 48 weeks; PR, pegylated interferon/ribavirin; RGT, response‐guided therapy; SPRINT‐2, Serine Protease Inhibitor Therapy 2; T8PR, telaprevir for 8 weeks and pegylated interferon/ribavirin; T12PR, telaprevir for 12 weeks and pegylated interferon/ribavirin.
