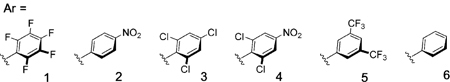
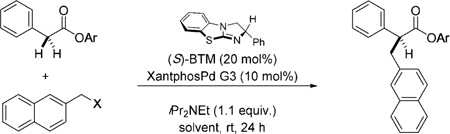
Table 1:
Optimization studies.
 | |||||
|---|---|---|---|---|---|
| Entry[a] | X | Ar | Solvent | Yield [%][b] | er[c] |
| 1 | OTs | 1 | THF | 0 | – |
| 2 | OAc | 1 | THF | 0 | – |
| 3 | OCO2tBu | 1 | THF | 0 | – |
| 4 | OP(O)(OEt)2 | 1 | THF | 5 | – |
| 5 | OP(O)(OPh)2 | 1 | THF | 68 | 97:3 |
| 6 | OP(O)(OPh)2 | 1 | 1,4-dioxane | 60 | 93:7 |
| 7 | OP(O)(OPh)2 | 1 | CH2Cl2 | 40 | 89:11 |
| 8 | OP(O)(OPh)2 | 1 | toluene | 85 (81) | 99:1 |
| 9 | OP(O)(OPh)2 | 2 | toluene | 82 | 94:6 |
| 10 | OP(O)(OPh)2 | 3 | toluene | 21 | – |
| 11 | OP(O)(OPh)2 | 4 | toluene | 0 | – |
| 12 | OP(O)(OPh)2 | 5 | toluene | 75 | 99:1 |
| 13 | OP(O)(OPh)2 | 6 | toluene | 0 | – |
Reactions performed on 0.1 mmol scale.
Yields determined by 1H NMR analysis using 1,2,4,5-tetramethylbenzene as an internal standard. Yields of isolated products given in parentheses.
Determined by HPLC analysis on a chiral stationary phase.
Ms= methane-sulfonyl, Pfp = pentafluorophenyl.