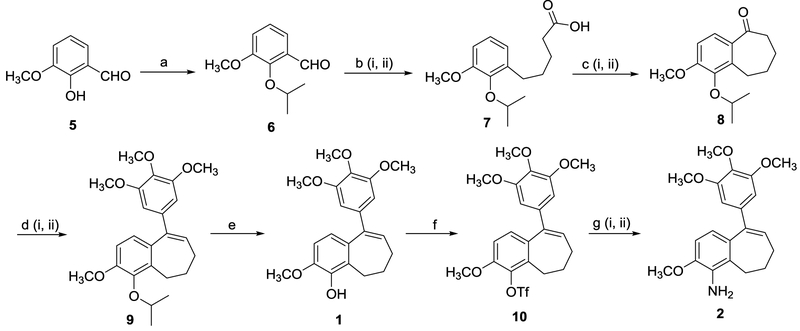
Scheme 2.
Modified synthetic routes to compounds 1 and 2. Reagents and reaction conditions: (a) compound 5 (1.0 eq.), K2CO3 (1.5 eq.), 2-iodopropane (2.0 eq.), DMF, 50–60 °C, 20 h, 98%. (b) (i) 3-(carboxypropyl)triphenylphosphonium bromide (1.5 eq.), potassium tert-butoxide (3.5 eq.), compound 6 (1.0 eq.), DMF, 0 °C to RT, 24 h; (ii) Pd-C (0.1 eq., 10 wt%), H2 balloon, CH3OH, 24 h, 85–94% (two steps). (c) (i) compound 7 (1.0 eq.), oxalyl chloride (2.0 eq.), DMF (0.2 eq.), CH2Cl2, RT, 2 h; (ii) SnCl4 (1.2 eq.), CH2Cl2, −10 °C, 40 min, 75–80% (two steps). (d) (i) 5-bromo-1,3,4-trimethoxybenzene (2.0 eq.), n-BuLi (2.0 eq.), THF, −78 °C, 30 min. (ii) compound 8 (1.0 eq.), −78 °C, 4 h, RT, 16 h, 84%. (e) compound 9 (1.0 eq.), boron trichloride (1.1 eq.), CH2Cl2, 0 °C, 2 h, 85–92%. (f) compound 1 (1.0 eq.), triethylamine (2.0 eq.), triflic anhydride (1.5 eq.), CH2Cl2, 0 °C to RT, 5 h, 80–92%. (g) (i) compound 10 (1.0 eq.), benzophenone imine (1.5 eq.), Cs2CO3 (1.5 eq.), palladium(II) acetate (0.1 eq.), racemic-BINAP (0.15 eq.), toluene, 110–115 °C, 36 h; (ii) 2 M HCl, RT, 1 h, THF, 79–93% (two steps).