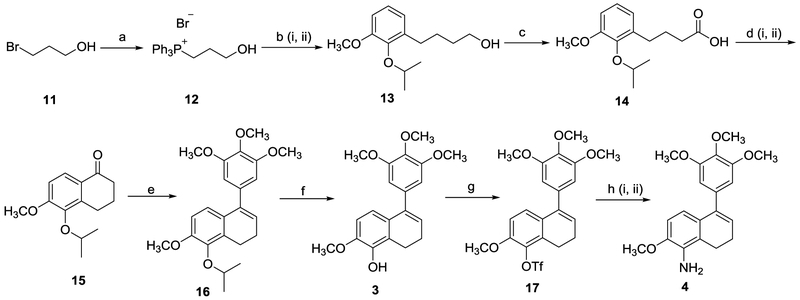
Scheme 4.
New synthetic routes to compounds 3 and 4. Reagents and reaction conditions: (a) compound 11 (2.0 eq.), triphenylphosphine (1.0 eq.), toluene, reflux, 24 h, 96%. (b) (i) compound 12 (1.5 eq.), n-BuLi (3.0 eq.), THF, 0 °C, 15 min; TMSCl (1.5 eq.), 0 °C, 30 min; compound 6 (1.0 eq.), 0 °C, 1 h, RT, 2 h (ii) Pd-C (0.1 eq., 10 wt%), H2 balloon, CH3OH, 24 h, 92% (two steps, compound 13). (c) compound 13 (1.0 eq.), Oxone® (1.5 eq.), IBX (0.3 eq.) ACN/H2O, 70 °C, 20 h, 71%. (d) (i) compound 14 (1.0 eq.), oxalyl chloride (2.0 eq.), DMF (0.2 eq.), CH2Cl2, RT, 2 h; (ii) SnCl4 (1.2 eq.), CH2Cl2, −10 °C, 40 min, 92% (two steps). (e) (i) 5-bromo-1,3,4-trimethoxybenzene (2.0 eq.), n-BuLi (2.0 eq.), THF, −78 °C, 30 min. (ii) compound 15 (1.0 eq.), −78 °C, 4 h, RT, 16 h, 65%. (f) compound 16 (1.0 eq.), boron trichloride (1.1 eq.), CH2Cl2, 0 °C, 3 h, 85%. (g) compound 3 (1.0 eq.), Triethylamine (2.0 eq.), triflic anhydride (1.5 eq.), 0 °C to RT, 5 h, 83–100%. (h) (i) compound 17 (1.0 eq.), benzophenone imine (1.5 eq.), Cs2CO3 (1.5 eq.), palladium(II) acetate (0.1 eq.), racemic-BINAP (0.15 eq.), toluene, 110–115 °C, 36 h; (ii) 2 M HCl, RT, 1 h, THF, 72% (two steps).