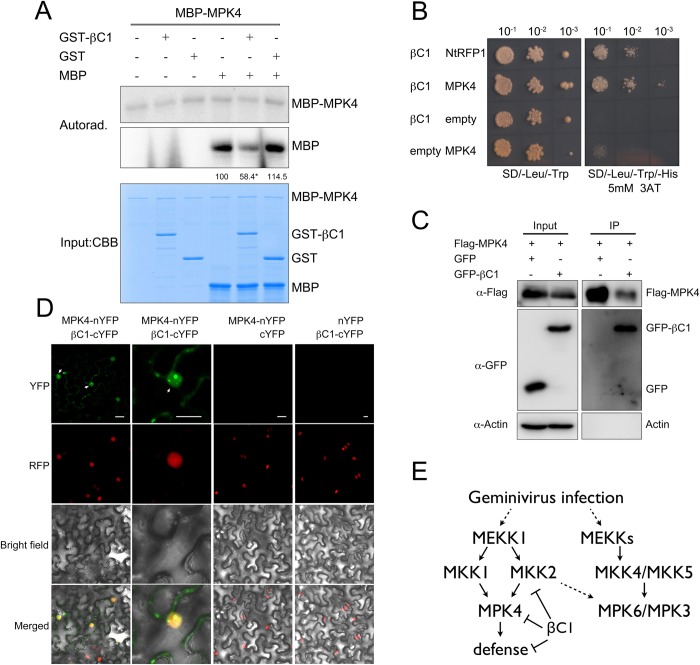
Fig 6. βC1 physically interacts with MPK4.
(A) In vitro immunocomplex kinase assays show that βC1 suppresses MPK4 activity. Different purified kinases, myelin basic protein (MBP) substrates, and [γ-32P] ATP were incubated with either GST-βC1 or GST protein. Proteins were resolved in SDS-PAGE and analyzed by autoradiography. Protein loading is shown by Commassie Brilliant Blue staining (CBB). Numbers indicate relative amount of phosphorylated MBP protein of three biological replicates. Asterisk indicates significant differences (p<0.05, Student’s t test). (B) Y2H assay shows that βC1 interacts with MPK4. Yeast transformant harboring different combinations of AD-βC1 and BD-MPK4 were spotted with 10-fold serial dilutions on SD/-Leu/-Trp medium and SD/-Leu/-Trp/-His containing 5mM 3-AT. βC1 and NtRFP1 serve as positive control. (C) Co-IP assay confirmed the in planta interaction between βC1 and MPK4. Samples before (Input) and after (IP) immunopurification were analyzed by immunoblot using anti-GFP and anti-Flag antibodies, actin serves as a control. (D) BiFC assay validates the interaction between MPK4 and βC1. RFP-H2B transgenic N. benthamiana leaves were infiltrated with A. tumefaciens harboring combinations of indicated constructs. Columns from left to right represent YFP fluorescence, RFP fluorescence, bright field and YFP/RFP/bright field overlay. Bars represent 20μm. (E) The proposed model of βC1 effect on MAPK cascade in virus defense. Geminivirus infection induces activation of MPK6/MPK3 and MPK4, βC1 blocks MAPK cascade regulated defense by inhibiting MKK2 and MPK4.