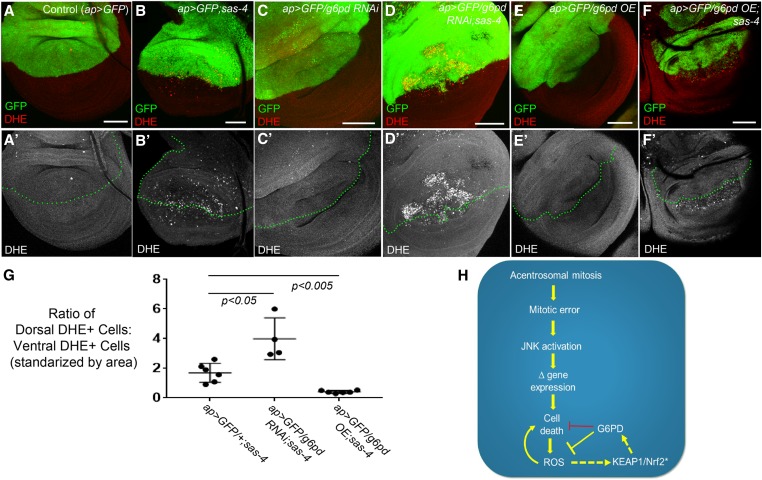
Figure 8.
G6PD buffers acentrosomal cells against ROS production. (A–F) Assessment of ROS levels in third-instar wing using DHE. (A-A’) ap>GFP alone does not induce ROS. (B-B’) ap>GFP also does not affect levels of ROS associated with centrosome loss (sas-4 homozygous mutant disc). (C-C’) g6pd knockdown in otherwise normal cells does not induce ROS production. (D-D’) However, g6pd knockdown does elevate ROS in sas-4 mutant cells above levels caused by centrosome loss alone. (E-E’) G6PD OE does not affect baseline ROS production in WT. (F-F’) G6PD OE significantly reduces levels of ROS in acentrosomal cells. Bar, 50 µm. All images are maximum-intensity projections. (G) Quantification of the effects of sas-4 and g6pd manipulations on ROS levels; see the Materials and Methods for a detailed description of these calculations. (H) Speculative model of the relationships between the relevant pathways, processes, and genes. Because G6PD buffers ROS levels in some cells and because ROS can contribute to cell death, G6PD can indirectly inhibit cell death (this indirect relationship is indicated by the red repression symbol). The activation of KEAP1/Nrf2 signaling indicated by “*” is inferred from increased ROS levels, which are known to activate KEAP1/Nrf2, and from increased expression of GstD1>GFP, which is regulated by KEAP1/Nrf2 (this inferred activity is indicated by the dashed arrow). Similarly, we infer that G6PD is upregulated by KEAP1/Nrf2 activity in acentrosomal cells because g6pd is a previously identified transcriptional target of that pathway. DHE, dihydroethidium; G6PD, glucose-6-phosphate dehydrogenase; OE, overexpression; RNAi, RNA interference; ROS, reactive oxygen species; WT, wild-type.