Figure 7.
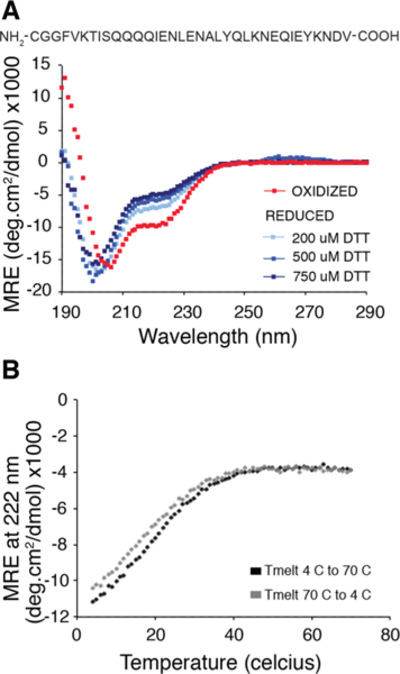
Marginal stability of the coiled-coil linker is shown using CD spectroscopy. (a) CD spectrum of the linker peptide (40 μM peptide in 10 mM NaPi buffer, pH 7) under air oxidized (red) vs reduced conditions (shades of blue). Helical character at 222 nm is only seen when the cysteine is oxidized indicating that the coiled-coil sequence is not sufficient to fold the linker at micromolar concentrations in the absence of tethering. (b) CD melting curve (at 222 nm) for the oxidized form of the peptide shows marginal helicity at room temperature and significant stabilization of the helix at lower temperatures. The melting curves are reversible (black, 4C to 70C and gray, 70C to 4C).
