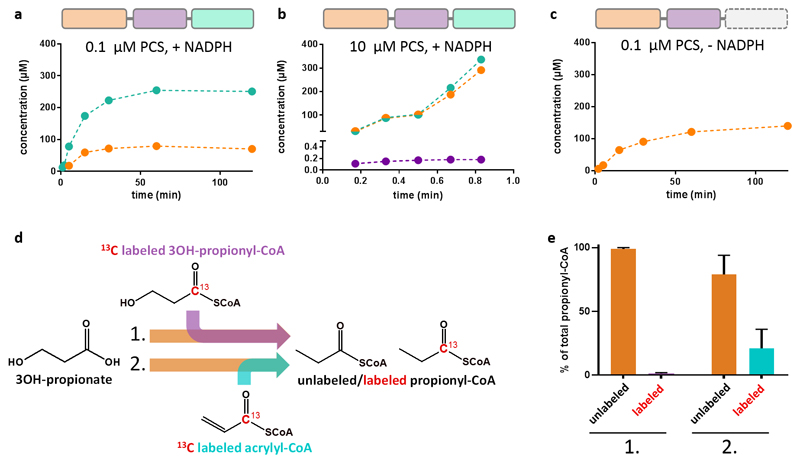
Figure 2. PCS sequesters the reactive intermediate acrylyl-CoA.
a, Time course of the overall reaction with 0.1 µM PCS, 800 µM CoA, 500 µM 3-hydroxypropionate, 800 µM ATP and 300 µM NADPH. Production of the 3-hydroxypropionyl-CoA intermediate (orange) and the final product propionyl-CoA (cyan) was observed. In contrast no free acrylyl-CoA was detectable. b, Time course of the reaction containing 10 µM PCS, 5 mM CoA, 5 mM 3-hydroxypropionate, 5 mM ATP and 5 mM NADPH. At these high enzyme concentrations acrylyl-CoA (purple) was detected at 0.18 µM during steady-state corresponding to 1.8% occupancy of reductase active sites. 3-Hydroxypropionyl-CoA und propionyl-CoA accumulate over time. c, as in a, but without NADPH. Again, formation of 3-hydroxypropionyl-CoA was observed, but not of free acrylyl-CoA. d, Isotopic labeling competition experiment containing unlabeled 3-hydroxypropionate and either 13C-labeled 3-hydroxypropionyl-CoA (experiment 1) or acrylyl-CoA (experiment 2). The reaction was started by the addition of PCS. Products were analyzed by LC-MS (see Supplementary Table 4 for detailed assay conditions). e, Results of the isotopic labeling competition experiment. Only 0.8 ± 0.4 % of propionyl-CoA was produced from exogenous 13C-labeled 3-hydroxypropionyl-CoA during steady state (experiment 1). Approximately every fifth propionyl-CoA (21 ± 15%) was formed from exogenous 13C-labeled acrylyl-CoA during steady-state (experiment 2). a – c, data of a representative single experiment. e, data mean ± s.d. (n=3).