Figure 2.
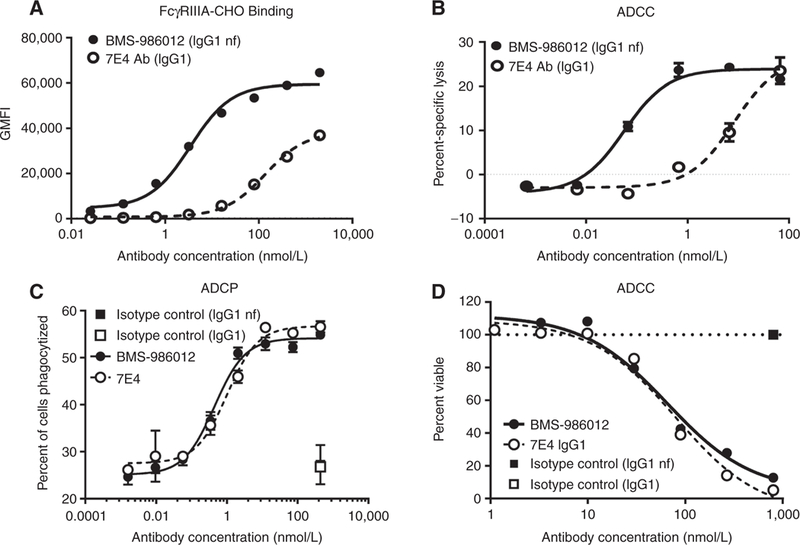
Comparison of FcγRIIIa binding and antibody-mediated effector functions of BMS-986012 and fucosylated parental antibody 7E4. Data are representative ofat least three independent experiments. A, FcγRIIIa receptor binding. BMS-986012demonstrates enhanced binding to CHOcells stablyexpressing human FcγRIIIa with binding EC5qs of 3.5 and 138 nmol/L for BMS-986012 ( ) and 7E4 (
) and 7E4 ( ), respectively. B, Enhanced BMS-986012-mediated FcγRIIIa-dependent ADCC activity in vitro. NK effector cells and DMS79 target cells were incubated with either BMS-986012 (
), respectively. B, Enhanced BMS-986012-mediated FcγRIIIa-dependent ADCC activity in vitro. NK effector cells and DMS79 target cells were incubated with either BMS-986012 ( ) or 7E4 (
) or 7E4 ( ). Median percent-specific lysis ± SD is plotted against antibody concentration. C, BMS-986012-mediated ADCP activity in vitro. Macrophage effector cells and DMS79 target cells were incubated with BMS-986012 (
). Median percent-specific lysis ± SD is plotted against antibody concentration. C, BMS-986012-mediated ADCP activity in vitro. Macrophage effector cells and DMS79 target cells were incubated with BMS-986012 ( ), 7E4 (
), 7E4 ( ), or isotype control antibodies IgG1nf (
), or isotype control antibodies IgG1nf ( ) and IgG1 (
) and IgG1 ( ). Macrophage-phagocytosed tumor cells were identified by flow cytometry as PKH26+ CD14+ double-positive events. The median percent of double-positive events ± SD is plotted against antibody concentration. D, BMS-986012-mediated CDC activity in vitro. DMS79 target cells were incubated with BMS-986012 (
). Macrophage-phagocytosed tumor cells were identified by flow cytometry as PKH26+ CD14+ double-positive events. The median percent of double-positive events ± SD is plotted against antibody concentration. D, BMS-986012-mediated CDC activity in vitro. DMS79 target cells were incubated with BMS-986012 ( ), 7E4 (
), 7E4 ( ), or isotype control antibodies IgG1nf (
), or isotype control antibodies IgG1nf ( ) and IgG1 (
) and IgG1 ( ) plus human complement. Cell viability was determined using CellTiter-Glo, and the percentage of viable cells is plotted against Ab concentration.
) plus human complement. Cell viability was determined using CellTiter-Glo, and the percentage of viable cells is plotted against Ab concentration.
