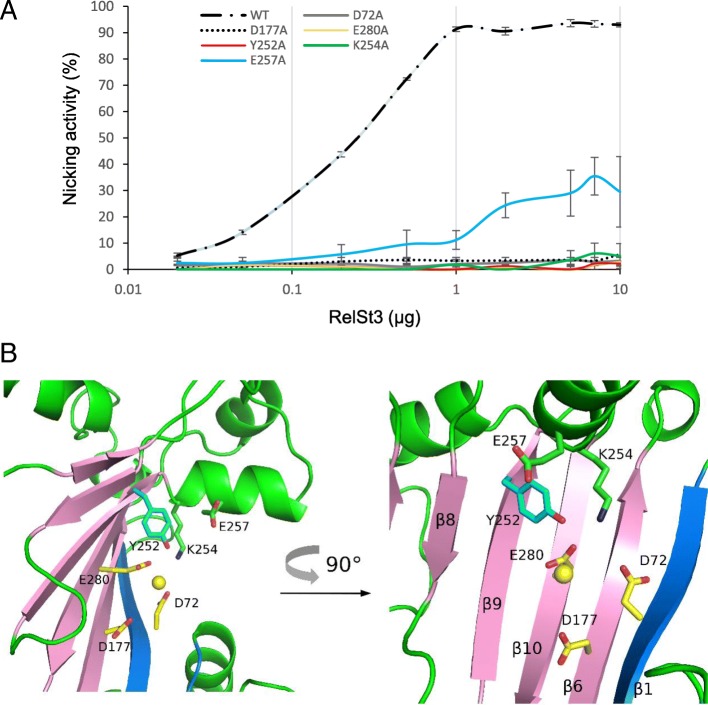
Fig. 7.
RelSt3 active site characterization. a Relaxation activity of RelSt3 point mutants. Dose-response relaxation activity was measured for the point mutants on the catalytic Tyr (Y252) in motif III, and on the adjacent K254 and E257 residues in motif III, and for the point mutants on the 3 conserved acidic residues involved in cation coordination (D72A, D177A and E280A). Relaxation experiments were performed on the pBR322-oriT substrate following the standard conditions described in Materials and Methods (Fig. 5c). The standard deviation of 3 replicates is shown. b Detailed structure of the homology model of RelSt3 active site. Cartoons are coloured as in Fig. 4. The conserved residues in the active site are represented as sticks and coloured as in Fig. 4. A 90 °C rotated representation is also shown