Multiple sclerosis (MS) is an inflammatory, demyelinating, and neurodegenerative disease with high prevalence between 20 to 50 y of age and a predominant 3:1 female:male ratio. Heterogeneous in its pathological and clinical manifestations (1), MS has been classified into relapsing–remitting and progressive forms. Primary progressive MS (PPMS) is defined as sustained disability from onset for 1 y in the absence of relapses (2). Secondary progressive MS, however, is characterized by relapses from onset where after several years the disease worsens with or without exacerbations, leading to marked disability progression (2). Beyond the complexities of the cellular and molecular pathophysiology of MS lies the human element. This is a costly disease where over 50% of patients, especially those with progressive MS, will lose their jobs and experience depression, cognitive decline, and gait disability. Based on genome-wide association studies (GWAS), MS susceptibility is linked to more than 230 genetic variants. These genes function in immune pathways and are expressed predominantly on T and B cells compared with microglia and astrocytes (3). Environmental factors also influence disease pathogenesis, including vitamin D deficiency (4), smoking (5), and exposure to Epstein–Barr virus.
How a disease with a predominant immune etiology leads to neurodegeneration, lack of repair, and progression has become one of the most significant questions in neuroimmunology. One of the first critical steps toward answering this will be untangling the basic mechanisms for progression and the biological and environmental factors that precipitate entry into the progressive phase. There are several theories which together with recent data are slowly illuminating this complex phase of the disease. One such hypothesis is that MS is not one disease but rather multiple autoimmune demyelinating syndromes with individual phenotypes that share similar underlying mechanisms and genetic variants. Another crucial question is whether progressive MS is prompted by a decrease in the natural compensatory mechanisms of brain reserve due to faulty repair processes or by sustained ongoing neuroinflammation, targeting neuroaxonal integrity and cells with the ability to repair. Thus far, several models of progression, coupled with research in MS tissue and animal models (6, 7), favor the latter where a multihit pathological cascade (8) causes faulty repair that worsens with increasing attacks and neuroaxonal dysfunction in normal-appearing white matter (NAWM) and cortical lesions. Molecularly, these events stem from an aberrant, sustained activation of microglia and astrocytes and a persistence of CD8 T cells in lesions, both of which appear to be a critical in the pathological evolution toward progression (9). Despite the clues into pathophysiology uncovered by GWAS, there has been no progress in our understanding of modifier genes contributing to neurodegeneration and lack of repair.
In PNAS, Nicaise et al. (10) show cellular senescence targeting neural precursor cells (NPCs) as a potential mechanism to explain the lack of repair in patients with PPMS. The authors reveal an increase in SOX2/p16ink4a-positive cells in the demyelinated, subcortical WM of PPMS patients, indicating that a population of SOX2+ human NPCs up-regulates the senescent marker p16 ink4a. These results are supported by data from inducible pluripotent stem cell (iPSC)-derived NPCs from PPMS patients, which express elevated p16 ink4a and SA-β-gal, another marker of senescence. This research was prompted by the authors’ past work demonstrating an intrinsic defect in PPMS NPCs for promoting myelination of oligodendrocyte progenitor cells (OPCs) through paracrine interaction (11). To identify molecular culprits underlying this phenotype, the authors studied the transcriptomes of NPCs from PPMS and control patients and found dysregulation of the mTOR pathway in PPMS NPCs that is reversed by treatment with rapamycin, a Food and Drug Administration-approved immunosuppressive agent indicated in the United States for prophylaxis of organ rejection. Rapamycin reduced senescent marker expression in PPMS NPCs, rescued OPC differentiation in the presence of PPMS NPCs, and decreased the expression of aging-associated proteins, indicating that these cell-autonomous defects can be reversed pharmacologically. Proteomic analysis of the iPSC-derived NPC secretome revealed high mobility group protein B1 (HMGB1) as a candidate gene differentially expressed by senescent NPCs, which the authors noted to be up-regulated by SOX2+ cells in PPMS patient brain tissue. Importantly, they provide experimental evidence indicating that HMGB1, secreted by senescent NPCs, negatively influences OPC differentiation. Altogether, the authors propose that SOX2+ cells in the WM of PPMS patients exert detrimental effects in the microenvironment of OPCs via paracrine interactions, leading to alteration of OPCs with myelinating potential.
The work performed here, with a series of elegant experiments using human cells rather than animal models, underscores a role for cellular senescence in MS as an important determinant for repair potential. These findings also represent a significant advance in our understanding of the molecular defects that may accompany NPCs in PPMS patients and point to required paracrine participation by SOX2+ cells in vivo for remyelination. Moreover, this work expands the list of molecules that mediate such defects in MS, including the Jagged1-Notch pathway (12), CD44 and proteoglycan, sonic hedgehog, and GLI1 (13, 14), and offers a therapeutically targetable, proof-of-principle mechanism in mTOR. Finally, these findings underline cellular aging as an additional obstacle that must be overcome when considering approaches to enhance remyelination, which unlike the promise observed in preclinical animal work (15) only shows modest results thus far in humans (16). Perhaps this lack of translational success suggests that individual heterogeneity in repair mechanisms and neurodegeneration among patients must be considered for personalized remyelinating and neurorestorative strategies. Spurred by these findings, additional efforts are needed to find biomarkers and endophenotypes of accelerated brain aging of individual PPMS patients to better select for clinical trials with mTOR inhibitors tailored for reverting NPC aging while protecting OPC maturation.
This work emphasizes the utility of patient-derived cells, like iPSCs, as powerful tools to investigate mechanisms of neurodegeneration (17). Compared with other neurological disorders, iPSCs are less frequently used to model MS, perhaps due to the traditional emphasis of studying immunopathogenesis rather than intrinsic neuronal and oligodendroglial mechanisms. As highlighted by this paper, there is a clear utility in using reductionist models to dissect the complexity of MS pathogenesis. In fact, it can be argued that these models may be more directly applicable to finding elusive CNS cell-autonomous defects, independent of neuronal susceptibility in cortical MS lesions, or oligodendrocyte heterogeneity in the human brain, which is difficult to recapitulate in animal models (17). To this end, several recent technological advances now enable analysis of human tissue at single-cell resolution. Leveraging such technology, Jäkel et al. (18) performed single-cell RNA sequencing of
In PNAS, Nicaise et al. show cellular senescence targeting neural precursor cells (NPCs) as a potential mechanism to explain the lack of repair in patients with PPMS.
MS brain tissue and uncovered a striking heterogeneity in oligodendrocytes in the human brain. Of these, only certain subpopulations of oligodendrocytes were altered in the NAWM of MS brains and accompanied by loss of OPCs in lesions and NAWM, which may explain poor repair in some patients. Besides oligodendrocytes, there is growing interest in other cells involved in MS pathology, namely astrocytes and microglia. Recent work utilizing MS patient-derived astrocytes demonstrates that variants in NF-κB are important for increased astrocyte-induced inflammation (19). Furthermore, microglia were recently shown to be involved in remyelination and oligodendrogenesis through expression of the lectin Chitinase 3-like-3 (Chi3l3), which is increased by IL-4 in mice. Chi3L1 and Chit1, the human paralogues of Chi3l3, were similarly found to regulate human oligodendrogenesis in vitro. Eradication of microglia Chi3l3 in an animal model of MS amounted to progressive disease, supporting the notion that lack of oligodendrogenesis or OPC maintenance contributes to the progressive phenotype (20).
Finding new molecules to avoid progression in MS will require studying underlying mechanisms of disease pathogenesis within the CNS. Past translational success and the current repertoire of FDA-approved immunomodifiers in MS relied on a steadfast dedication to identifying fundamental steps of immunopathogenesis in T and B cells. Similar work is underway in the investigation of repair and neurodegenerative mechanisms in MS, focusing chiefly on neurons, oligodendrocytes, and endogenous stem cells. To advance these efforts, new technologies to model the disease with patient-derived cells are being deployed. Human 3D culture techniques are gaining traction for modeling unique aspects of the disease, as there are several laboratories generating minibrain organoids with oligodendrocytes (21). Additionally, patient-derived monocytes differentiated into microglia represent another robust modeling tool, especially since they conserve the genetic susceptibly of native microglia from MS (22). Combining such emerging tools with novel pathophysiologic ideas will bolster our ability to understand the basic principles of neurodegeneration and repair in MS to an extent impossible with animal models.
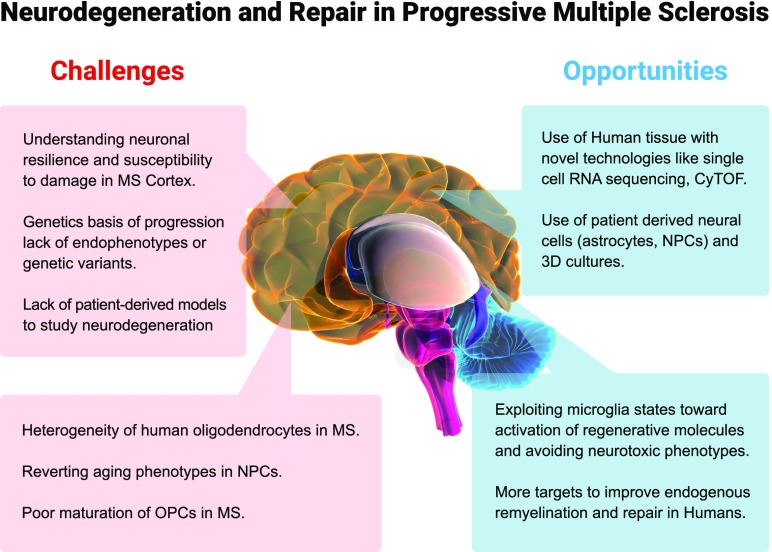
Undoubtedly, this work, together with other recent studies, is marking a new age of prosperity for progressive MS research at the fundamental level with exciting opportunities but also challenges that await (Fig. 1). Supported by a well-rewarded principle of translation in MS immunological research—that an unbiased focus on basic mechanistic research of pathogenesis and careful selection of the most promising candidates will be most poised to benefit patients (23)—these advances will hopefully unveil new pathological mechanisms that yield powerful therapies for progressive MS.
Fig. 1.
Conceptual framework for challenges and opportunities to target neurodegeneration and repair in progressive MS based on recent discoveries.
Footnotes
The author declares no conflict of interest.
See companion article on page 9030.
References
- 1.Howard J, Trevick S, Younger DS. Epidemiology of multiple sclerosis. Neurol Clin. 2016;34:919–939. doi: 10.1016/j.ncl.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 2.Lublin FD, et al. Defining the clinical course of multiple sclerosis: The 2013 revisions. Neurology. 2014;83:278–286. doi: 10.1212/WNL.0000000000000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sawcer S, et al. International Multiple Sclerosis Genetics Consortium; Wellcome Trust Case Control Consortium 2 Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 2011;476:214–219. doi: 10.1038/nature10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pierrot-Deseilligny C, Souberbielle JC. Vitamin D and multiple sclerosis: An update. Mult Scler Relat Disord. 2017;14:35–45. doi: 10.1016/j.msard.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 5.Briggs FBS, et al. Multiple sclerosis risk factors contribute to onset heterogeneity. Mult Scler Relat Disord. 2019;28:11–16. doi: 10.1016/j.msard.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Kutzelnigg A, et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain. 2005;128:2705–2712. doi: 10.1093/brain/awh641. [DOI] [PubMed] [Google Scholar]
- 7.Rasmussen S, et al. Persistent activation of microglia is associated with neuronal dysfunction of callosal projecting pathways and multiple sclerosis-like lesions in relapsing–remitting experimental autoimmune encephalomyelitis. Brain. 2007;130:2816–2829. doi: 10.1093/brain/awm219. [DOI] [PubMed] [Google Scholar]
- 8.Imitola J, Chitnis T, Khoury SJ. Insights into the molecular pathogenesis of progression in multiple sclerosis: Potential implications for future therapies. Arch Neurol. 2006;63:25–33. doi: 10.1001/archneur.63.1.25. [DOI] [PubMed] [Google Scholar]
- 9.Machado-Santos J, et al. The compartmentalized inflammatory response in the multiple sclerosis brain is composed of tissue-resident CD8+ T lymphocytes and B cells. Brain. 2018;141:2066–2082. doi: 10.1093/brain/awy151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicaise AM, et al. Cellular senescence in progenitor cells contributes to diminished remyelination potential in progressive multiple sclerosis. Proc Natl Acad Sci USA. 2019;116:9030–9039. doi: 10.1073/pnas.1818348116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicaise AM, et al. iPS-derived neural progenitor cells from PPMS patients reveal defect in myelin injury response. Exp Neurol. 2017;288:114–121. doi: 10.1016/j.expneurol.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 12.John GR, et al. Multiple sclerosis: Re-expression of a developmental pathway that restricts oligodendrocyte maturation. Nat Med. 2002;8:1115–1121. doi: 10.1038/nm781. [DOI] [PubMed] [Google Scholar]
- 13.Back SA, et al. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat Med. 2005;11:966–972. doi: 10.1038/nm1279. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Imitola J, Rasmussen S, O’Connor KC, Khoury SJ. Paradoxical dysregulation of the neural stem cell pathway sonic hedgehog-Gli1 in autoimmune encephalomyelitis and multiple sclerosis. Ann Neurol. 2008;64:417–427. doi: 10.1002/ana.21457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Najm FJ, et al. Drug-based modulation of endogenous stem cells promotes functional remyelination in vivo. Nature. 2015;522:216–220. doi: 10.1038/nature14335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cadavid D, et al. RENEW Study Investigators Safety and efficacy of opicinumab in acute optic neuritis (RENEW): A randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2017;16:189–199. doi: 10.1016/S1474-4422(16)30377-5. [DOI] [PubMed] [Google Scholar]
- 17.Hollingsworth EW, et al. iPhemap: An atlas of phenotype to genotype relationships of human iPSC models of neurological diseases. EMBO Mol Med. 2017;9:1742–1762. doi: 10.15252/emmm.201708191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jäkel S, et al. Altered human oligodendrocyte heterogeneity in multiple sclerosis. Nature. 2019;566:543–547. doi: 10.1038/s41586-019-0903-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ponath G, et al. Enhanced astrocyte responses are driven by a genetic risk allele associated with multiple sclerosis. Nat Commun. 2018;9:5337. doi: 10.1038/s41467-018-07785-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Starossom SC, et al. Chi3l3 induces oligodendrogenesis in an experimental model of autoimmune neuroinflammation. Nat Commun. 2019;10:217. doi: 10.1038/s41467-018-08140-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marton RM, et al. Differentiation and maturation of oligodendrocytes in human three-dimensional neural cultures. Nat Neurosci. 2019;22:484–491. doi: 10.1038/s41593-018-0316-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryan KJ, et al. A human microglia-like cellular model for assessing the effects of neurodegenerative disease gene variants. Sci Transl Med. 2017;9:eaai7635. doi: 10.1126/scitranslmed.aai7635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yednock TA, et al. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature. 1992;356:63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]