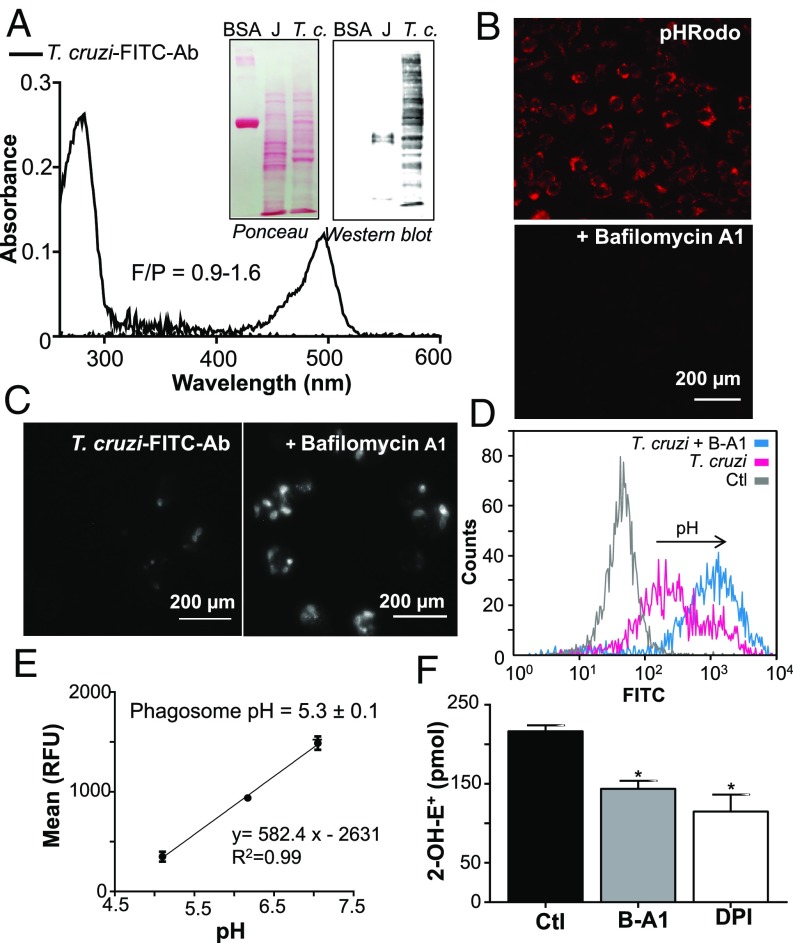
Fig. 6.
Intraphagosomal pH and O2•− permeation toward T. cruzi. (A) Absorption spectra of the purified FITC-labeled anti-T. cruzi antibodies (T. cruzi-FITC-Ab). (Inset) Specificity of FITC-labeled anti-T. cruzi antibodies assayed by Western blot using T. cruzi epimastigotes (T.c.) and macrophage extracts (J, 50 µg). (B) Macrophages (J774A.1) were incubated with pHrodo-Red (100 µg/mL) in the presence or absence of B-A1, and acidic phagosomes were visualized (red spots) by fluorescence microscopy. (Magnification: 400×.) (C) Macrophages were infected in the presence of T. cruzi-FITC-Ab with or without B-A1 (0.15 µM) for 10 min at 37 °C. Noninternalized parasites were removed, and cells were incubated for 15 min at 37 °C to allow phagosome acidification. (Magnification: 400×.) Increase in FITC fluorescence is detected in B-A1–treated cultures. (D) Flow cytometry quantification of macrophage FITC fluorescence. B-A1 was used as positive control (maximal FITC fluorescence); arrow indicates increase in fluorescence. (E) Calibration curve of FITC fluorescence mean vs. pH obtained as described in Materials and Methods. Phagosome pH was obtained by interpolating the T. cruzi fluorescence mean obtained in D in the calibration curve. (F) DHE-preloaded trypomastigotes were used to infect macrophages (2 h) in the presence or absence of 0.15 µM B-A1 or 100 µM DPI, and 2-OH-E+ was quantified by HPLC. Results are expressed as picomoles of 2-OH-E+ per 5 × 106 macrophages and represent the mean ± SEM of three samples; *P < 0.05. Ctl, control; RFU, relative fluorescence unit.