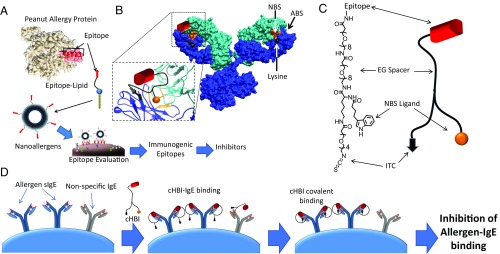
Fig. 1.
cHBIs are designed to selectively and irreversibly bind sIgEs and inhibit cellular degranulation. (A) Cartoon demonstration of the nanoallergen assay system for evaluating potential immunogenic epitopes. Potential epitopes of allergen proteins are synthesized as linear peptides conjugated to lipid tails (epitope-lipid). Epitope-lipids are then incorporated during liposome synthesis to produce nanoallergens. Nanoallergens are used to screen for epitope immunogenicity in cellular degranulation assays. (B) cHBI design. cHBIs were synthesized to display an immunogenic epitope that binds to the ABS of sIgEs, a NBS ligand that binds to the NBS of sIgEs, and an amine/lysine-reactive moiety (ITC) to facilitate irreversible binding to sIgEs. Representative crystal structure of an Ig with the ABS, NBS (orange), and a lysine proximal to NBS (black) is shown. The zoomed-in Fab region describes cHBI’s bivalent NBS and ABS binding, which promotes covalent conjugation to the proximal lysine. (C) Chemical structure of cHBI. The immunodominant epitope, NBS ligand, and ITC moiety are linked using flexible EG linkers to facilitate bivalent binding and covalent bond formation to sIgEs. (D) Schematic illustration of allergy inhibition by cHBIs. After initial bivalent binding to ABS and NBS, cHBI forms a covalent bond with sIgEs via ITC cross-linking of the nearby lysine, thereby inhibiting cellular degranulation.