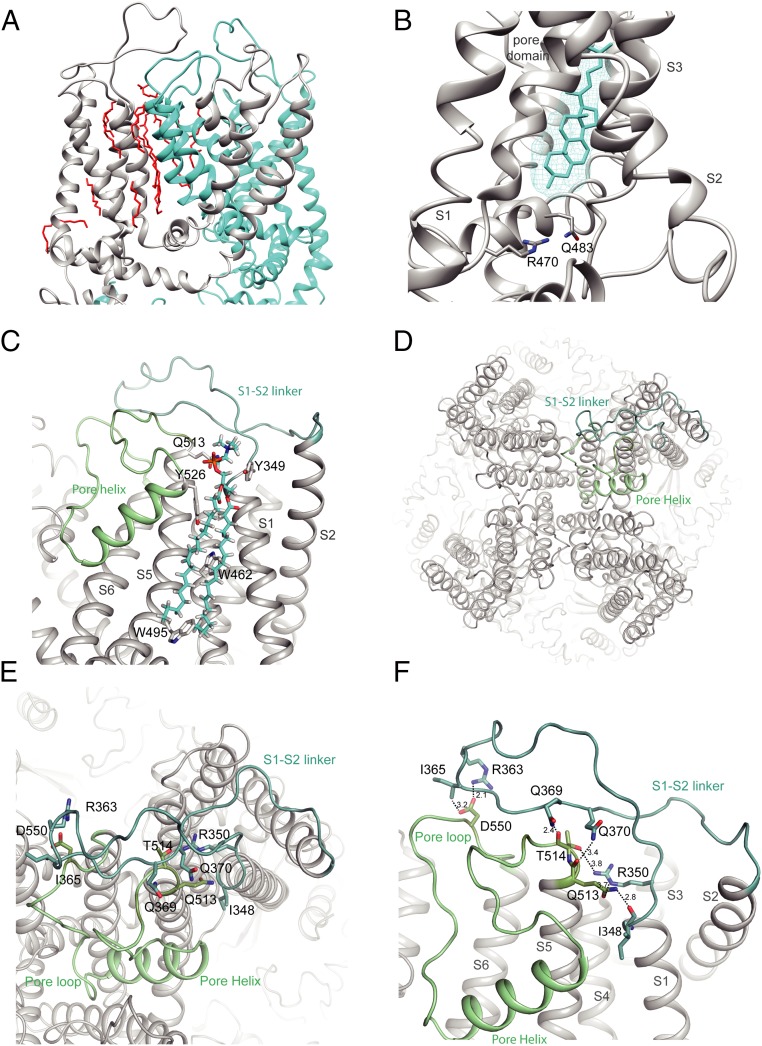
Fig. 2.
S1-S2 linker position in TRPV5. (A) Expanded view of the lipid densities observed within the pore domain. Two monomers are shown and individually colored. Lipids are in red. (B) Zoom of the resident lipid in the vanilloid pocket. The density of the lipid is visualized with cyan mesh, and the fitted acyl chain is in cyan. The side chains of residues important in phosphatidylinositol binding in TRPV1 are shown. (C) Zoom-in view of the interaction formed by the S1-S2 linker, annular lipid, and pore domain. The side chains of residues interacting with the lipid are shown. (D) Top view of TRPV5 tetramer showing the positions of the S1-S2 linker (light teal) and the S5-P-S6 domain (pale green). (E and F) Zoom-in view of the intersubunit interface formed by the S1-S2 linker and the pore helix. Putative hydrogen bonds and electrostatic interactions are shown as dashed lines. Side chains of interacting residues are shown as sticks for both, and interatomic distances (F) between side chains are depicted.