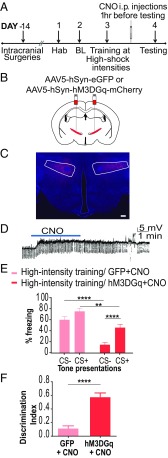
Fig. 3.
Increasing cellular activity in the ZI prevents fear generalization. (A) Experimental protocol for chemogenetic activation. Two weeks after intracranial injection of the control or DREADD virus, animals were conditioned to high-threat intensities. The next day, CNO was administered i.p. 1 h before testing fear generalization. BL, baseline; Hab, habituation. (B) Wild-type animals were injected with either the control virus (AAV5-hSyn-EGFP) or excitatory DREADDs (AAV5-hSyn-hM3DGq-mCherry) at −1.52 mm posterior to the bregma. (C) Representative image of the ZI targeted with intracranial infusions of DREADD-expressing mCherry viruses (in red) and Hoechst-stained nuclei (in blue). (Scale bar: 100 μm.) (D) Patch-clamp recording of hSyn-hM3DGq-mCherry–expressing cells in the ZI showing membrane depolarization during CNO exposure. (E) Training using high-intensity foot-shock causes fear generalization, as seen by high freezing to both CS+ and CS−. Chemogenetic activation of the ZI (hM3DGq+CNO) resulted in a significant decrease in the fear response to CS+ as well as CS− compared with controls (GFP+CNO). (F) Chemogenetic activation of the ZI (hM3DGq+CNO) resulted in a better ability to discriminate between the CS+ and the CS−. **P < 0.01; ****P < 0.0001. Data are represented as mean ± SEM.