Figure 4. Weak Binding of BID BH3 at the Canonical Hydrophobic Groove of BOK Promotes Membrane Permeabilization at Specific Dosing Combinations.
(A) Unstapled and stapled BH3 peptides. Residues highlighted in black are identical between BID and BOK BH3. X, pentenyl-Ala; B, norleucine.
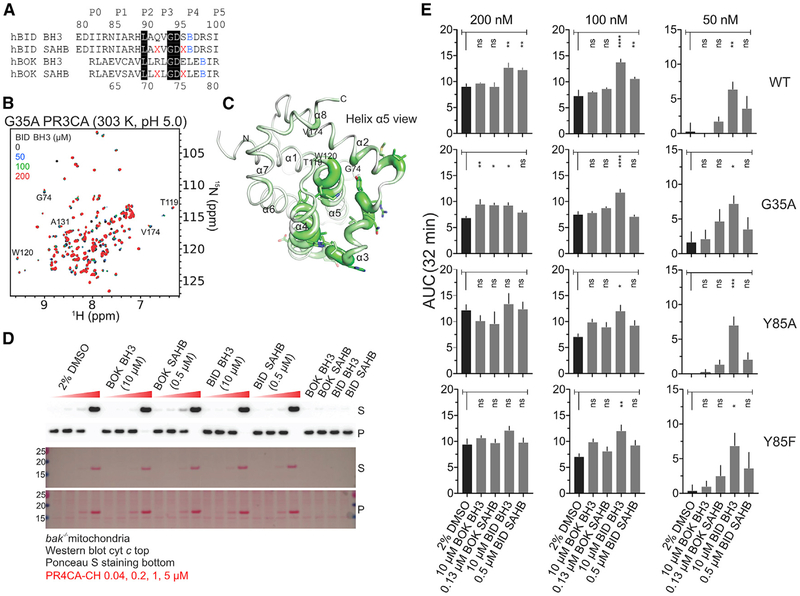
(B) Overlay of 1H-15N TROSY spectra of 15N-labeled G35A PR3CA titration with BID BH3 at 303 K and pH 5.0.
(C) CSP magnitudes in G35A PR3CA induced by 200 μM BID BH3 at 303 K and pH 5.0 (Figure S4B) were plotted over the cartoon putty representation of WT PR3CA. Putty thickness correlates with CSP magnitude.
(D) MOMP assay of purified bak−/− mitochondria by WT PR4CA-CH in the presence of unstapled and stapled BH3 peptides and the DMSO vehicle control for monitoring cyt c release and protein loading.
(E) Combined average and SE of AUC at 32 min for WT and mutant PR4CA-CH in the presence of unstapled and stapled BH3 peptides and DMSO vehicle control for the normalized LUVP in Figure S4G. Data were corrected for LUVP induced by peptides and vehicle alone (Figure S4F). Peptide concentrations were empirically selected to induce low or no LUVP and MOMP in the absence of BOK.
See also Figure S4.