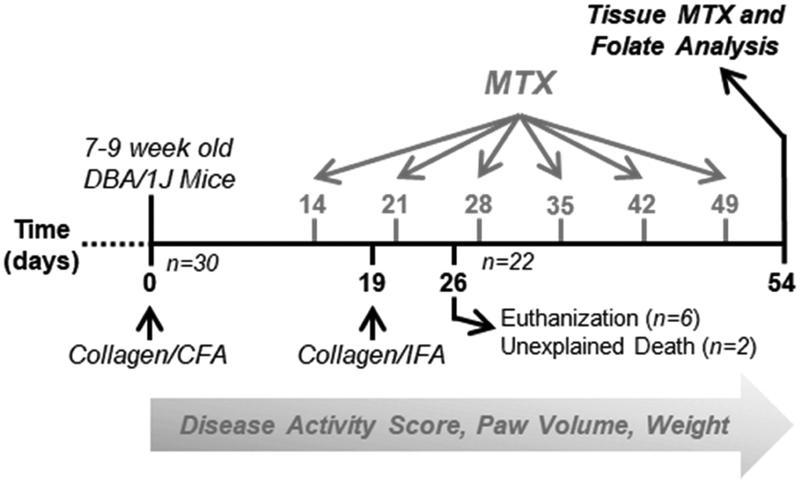
Fig. 1. Diagram of disease induction and MTX treatment study design.
DBA/1J mice were split into 3 groups including a healthy control group, a CIA disease control group, and a MTX treatment group. The CIA disease control group of MTX treatment group underwent the CIA disease induction protocol including an initial injection of collagen/CFA at day 0 and a booster injection of collagen/IFA at day 19. The MTX treatment group were further stratified into 4 subgroups and administered subcutaneous MTX at doses of 2, 10, 20, or 50 mg/kg weekly starting on day 14 for a total of 6 doses. Disease activity scores, paw volume measurements, and animal weight were collected throughout the study. At day 54 mice were euthanized and tissues were collected for MTX and folate analysis. Eight mice were lost during the study and not included in the terminal analysis.