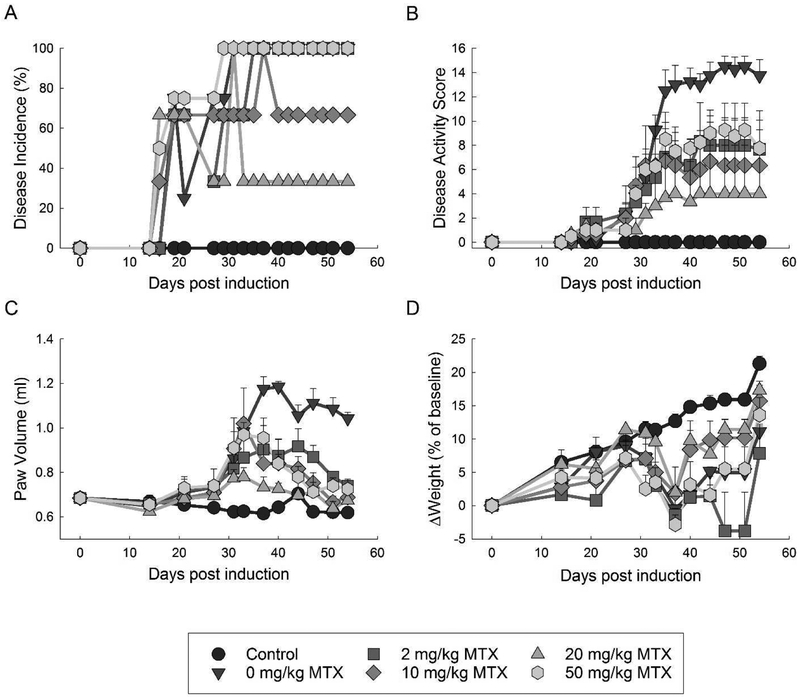
Fig. 2. Effect of MTX on disease incidence and activity in the CIA mouse model.
Disease incidence and activity were compared amongst control mice and mice undergoing the CIA protocol treated with weekly subcutaneous MTX at doses of 0, 2, 10, 20, or 50 mg/kg. Groups were compared based on the incidence of arthritis (A), disease activity (B), paw volume (C), and change in weight from baseline (D). Parameters were measured over the 54-day experimental period and the resulting mean±S.E.M. for each experimental group is presented.