Figure 21.
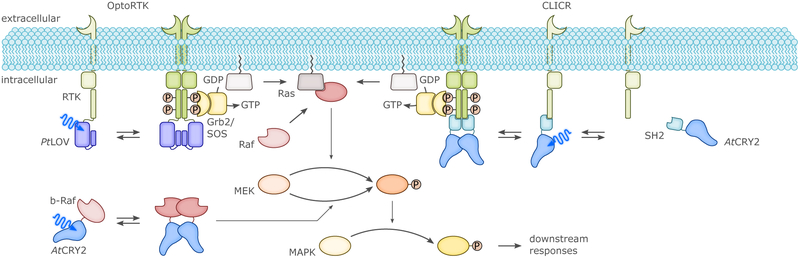
Receptor tyrosine kinase (RTK) signaling was subjected to BL-dependent optogenetic control as exemplarily illustrated for the MAPK/ERK pathway. In several approaches,190,311,312 OptoRTKs were constructed by appending an associating photoreceptor, e.g., the LOV domain of P. tricornutum aureochrome, to the intracellular C terminus of an RTK. BL then induced homodimerization of the chimeric receptor and activation of the downstream signaling cascade. In the CLICR strategy,315 endogenous RTKs could be activated upon BL exposure via an adapter protein consisting of AtCRY2 and an SH2 domain that specifically binds to the C termini of RTKs. The MAPK/ERK pathway was also targeted at lower tiers.316,317 On the one hand, the Raf kinase can be activated by recruiting it in BL-dependent manner to the plasma membrane (not shown). On the other hand, the B-Raf isoform can be activated away from the membrane in the cytosol by homodimerization or association with the isoform c-Raf. To optogenetically control these processes, the BL-dependent oligomerization of AtCRY2 or its interaction with AtCIB1 was harnessed.