Abstract
Diabetic nephropathy (DN) is the leading cause of end-stage renal disease (ESRD). Many trials have shown that Abelmoschus manihot could further improve proteinuria and protect kidney function in patients with DN when added to a renin-angiotensin system (RAS) blocker. A systematic assessment of the efficacy and safety of A. manihot in DN is essential. Eight electronic databases were searched to identify eligible trials published from inception to December 2017. The Cochrane Risk of Bias Tool was used to evaluate the methodological quality of eligible studies. Seventy-two studies with 5,895 participants were identified. The methodological quality of included studies was generally low. The results indicated that, compared to a RAS blocker, combined treatment of A. manihot with a RAS blocker was more effective for 24h urinary protein (24h UP) (mean difference [MD], -0.39 [95% confidence interval [CI], -0.46 to -0.33] g/d; P<0.00001), urinary albumin excretion rate (UAER)(MD, -19.90 [95% CI, -22.62 to -17.18] μg/min; P<0.00001), 24h UP reduction rate (risk ratio [RR], 1.43; 95% CI, 1.26-1.63; P<0.00001), normalization of UAER (RR, 1.48; 95% CI, 1.29-1.70; P<0.00001), and serum creatinine (SCr) (MD, -7.35 [95% CI, -9.95 to -4.76] umol/L; P<0.00001). None of these trials reported the ESRD rate. No statistically significant difference occurred between A. manihot combined with a RAS blocker and a RAS blocker alone in estimated glomerular filtration rate (eGFR) (MD, 4.43 [95% CI, -1.68 to 10.54] mL/min; P=0.16). A. manihot did not increase the rates of adverse drug events. A. manihot in addition to a RAS blocker was effective and safe to further improve proteinuria and protect kidney function in patients with DN. However, due to the generally low methodological quality, significant heterogeneity, and publication bias, high-quality randomized controlled trials are required to confirm these findings before the routine use of A. manihot can be recommended.
1. Introduction
Approximately 20% to 40% of patients with diabetes mellitus (DM) will develop diabetic nephropathy (DN) [1]. Type 2 diabetes mellitus (T2DM) is the most common cause of chronic kidney disease (CKD) and end-stage renal disease (ESRD) in the developed world and is the second leading cause of ESRD after primary glomerular disease in China [2–4]. DM and CKD are independent risk factors of all-cause mortality as well as cardiovascular death [5, 6]. Diabetic kidney disease (DKD) poses the highest risk for death compared to DM or CKD alone [5, 6].
Management of DN requires a multifaceted approach, including a combination of lifestyle modifications and pharmacologic intervention. The effectiveness of current interventions remains limited given the number of patients who continue to have progression of their renal dysfunction, despite blood pressure and glycemic control, and the use of existing renin-angiotensin system (RAS) blockers. Retrospective analyses of clinical studies concerning DN demonstrate a strong relationship between the magnitude of albuminuria reduction and slowing of CKD progression as well as reduced cardiovascular event rates [7–12]. An angiotensin-converting enzyme inhibitor (ACEI) combined with an angiotensin receptor blocker (ARB) is not recommended due to the high risk of hyperkalemia and/ or acute kidney injury as well as no benefit in altering the natural history of DN [1, 10]. Recently, mineralocorticoid receptor antagonist (MRA) in addition to an ACEI/ARB treatment has been studied as a novel approach to further prevent the progression of DN. A meta-analysis by Mavrakanas et al. [13] reported that combined treatment with an ACEI/ARB and an MRA was effective in decreasing albuminuria compared to standard treatment with an ACEI/ARB in DN but increased the risk of hyperkalemia. Therefore, there is an urgent need for a new pharmacologic agent that could be effective and safe to further improve proteinuria and prevent the progression of DN.
Traditional Chinese medicine (TCM) has shown promising effects on the control of proteinuria, protection of renal function, and improvements in patients' clinical symptoms [14]. Abelmoschus manihot has been in use for CKD in China for hundreds of years. Huangkui capsule, a single medicament of TCM extracted from the dry corolla of A. manihot, has been approved by China's State Food and Drug Administration (SFDA) for the treatment of chronic nephritis since 1999. A. manihot can ameliorate proteinuria and protect kidney function in patients with CKD, such as DN, immunoglobulin A nephropathy (IgAN), and membranous nephropathy, and is currently considered an important adjuvant therapy for CKD [15–20]. The major biologically active constituents are total flavones of A. manihot (TFA) [21]. Mechanistic studies applying A. manihot to the treatment of CKD suggest that the major effects are associated with improved immunological reaction, inflammation, renal fibrosis, and renal tubular epithelial injury [14, 22]. The results of previous meta-analyses preliminarily suggest that A. manihot could improve proteinuria and protect kidney function in patients with DN [16–19]. However, the evidence was very limited on the effect of A. manihot for DN due to a limited number of trials included, with poor methodological quality. A lot of novel data evaluating A. manihot in DN have been recently published. Therefore, we systematically analyzed the evidence on A. manihot in addition to a RAS blocker therapy in DN, focusing on its effect in albuminuria.
2. Methods
2.1. Data Sources and Searches
This systematic review was reported in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [23]. See File S1 in the Supplementary Material for the PRISMA 2009 checklist for this article. The review protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO registration no. CRD42018087182, available at http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42018087182). The Cochrane Central Register of Controlled Trials (CENTRAL) on the Cochrane Library, PubMed, EMBASE, Chinese National Knowledge Infrastructure database (CNKI), Chinese Biomedical Literature database (CBM), Chinese Scientific Journal database (VIP), and Wan Fang database were searched to identify eligible trials published from inception to December 15, 2017. Ongoing registered clinical trials were searched at ClinicalTrials.gov (https://www.clinicaltrials.gov). The articles were not restricted based on language. All included studies were subjected to the same quality assessment.
The search terms were as follows: Flos Abelmoschus manihot, Abelmoschus manihot, Abelmoschus moschatus Medicus, Abelmoschus, okra, Huangkui, Huangkui capsule, huangshukui, diabetic nephropathy, diabetes mellitus, diabetic, kidney disease, renal disease, diabetic kidney disease, diabetic renal disease, albuminuria, randomized controlled trial, controlled clinical trial, randomized, randomly, and trial. See File S2 in the Supplementary Material for an example of the full electronic search strategy. Two authors (L. W. Shi and M. Z. Zhang) performed independently the literature search. Disagreements were resolved by discussing with a third party (Q. Ni and L. Feng).
2.2. Study Selection
Eligible trials were listed and assessed independently by two reviewers (L. W. Shi and M. Z. Zhang) using predefined inclusion criteria. Studies were included if they met the following criteria: (1) it was randomized controlled design; (2) patients were with type 1 or type 2 DM and DN (defined as at least 30 mg of albuminuria in a 24h urine collection or urinary albumin excretion rate (UAER) of at least 20 μg/min); (3) participants should have received an ACEI or an ARB throughout the study as standard treatment. To evaluate the effect of concomitant A. manihot, a subset of patients in each study should also receive A. manihot in addition to standard RAS blockade; (4) the primary outcome measures included 24-h urinary protein (24h UP), ESRD rate and estimated glomerular filtration rate (eGFR). The secondary outcome measures were UAER, improvements in 24h UP reduction rate (defined as the proportion of 24h UP decrease in protein excretion ≥50% of the baseline at the end of the study), normalization of UAER (defined as the proportion of UAER <20 μg/min upon study completion), serum creatinine (SCr) and adverse drug events (ADEs); (5) the studies included available and relevant data; and (6) the studies were not restricted based on publication language.
Excluded from the meta-analysis were duplicated publications, studies with unavailable or incorrect data, and articles not reporting outcomes of interest. Also excluded were studies enrolling fewer than 10 participants, quasi-randomized controlled trials (e.g., allocation using alternation, the sequence of admission, case record numbers), and nonrandomized controlled clinical trials. Studies using combination RAS blockers as background therapy or A. manihot coupled with any other TCM drugs were excluded to avoid confounding information.
2.3. Data Extraction and Quality Assessment
Two authors (X. W. Li and Y. N. Yang) independently extracted information on the patients as well as on the methods, interventions, outcomes, and results using a predesigned data extraction form. The data extraction form included the following items: name of first author, year of publication, total number and number in both groups, gender and mean age, baseline characteristics, method of randomization, allocation concealment, incomplete outcome data, selective reporting, blinding, interventions, and outcomes.
The methodological quality of randomized controlled trials (RCTs) was independently assessed by two authors (M. Z. Zhang and Y. Y. Zhang) via the Cochrane Risk of Bias Tool [24]. Each study was respectively categorized as “low risk of bias”, “high risk of bias”, or “unclear risk of bias”. Authors were contacted by e-mail to obtain further data and verify the methodological quality when necessary. The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology was used to assess the quality of the evidence of each outcome. Any disagreement was settled by mutual discussion with a third author (Q. Ni and L. Feng).
2.4. Data Synthesis and Analysis
Dichotomous outcomes were pooled using risk ratio (RR) and 95% confidence intervals (CIs) and continuous outcomes were pooled using mean difference (MD, defined as the difference between study groups at the end of study) and 95% CIs. A random-effects model was used to pool the data. Statistical heterogeneity was assessed with the I-square (I2) statistic [25]. The I2 statistic of ≤50% referred to low statistical heterogeneity, while >50% was considered as substantial statistical heterogeneity. Publication bias was performed and evaluated using funnel plots, if the group included >10 trials [26]. Sensitivity analysis was assessed by excluding lower quality trials and repeating the meta-analyses to examine the effects of these study subgroups. We had no prespecified plan of subgroup analysis. Meta-analysis was performed by using Review Manager Version 5.3. All tests were 2-tailed, and P<0.05 was considered statistically significant.
3. Results
3.1. Included Studies and Trial Characteristics
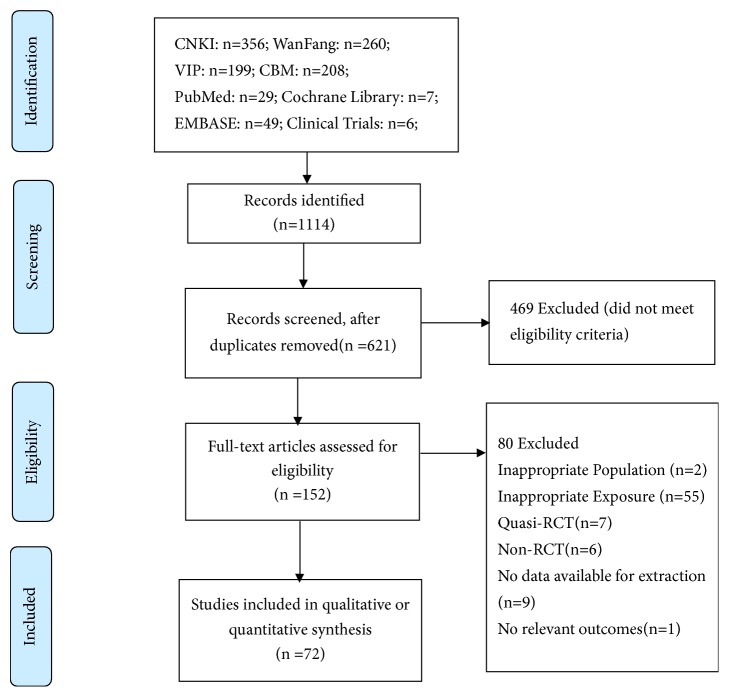
A flow diagram of study selection is shown in Figure 1. During the initial electronic search, 1,114 articles were identified, of which 962 were excluded including duplicates and irrelevant studies. The full texts of the selected 152 trials were retrieved, and after detailed evaluation, 72 RCTs [27–98] were finally selected for meta-analysis; of these, 28 met the inclusion criteria from 4 previous meta-analyses [16–19]. Authors were contacted by e-mail for additional outcome data; however, no reply was received.
Figure 1.
Flow diagram of study selection.
The baseline characteristics of DN patients are presented in Table 1. The 72 studies included a total of 5,895 patients followed-up from 4 to 24 weeks. The treatment and control groups consisted of 3,000 and 2,895 patients, respectively. Sample size of the included trials ranged from 40 to 200. The mean age reported for participants in these studies ranged from 36 to 69 years, and the proportion of males ranged from 33.3% to 69.2%. The average baseline protein level in urine was 1.94 g/d (0.14-6.2 g/d). The median follow-up for 24h UP was 12 weeks. A. manihot in the form of a Huangkui capsule (Jiangsu SuZhong Pharmaceutical Group Co., Ltd.) was given orally at 3.0 g 3 times daily in one trial [27], orally at 2.5 g 3 times daily in 67 trials [28–41, 43–47, 49–64, 66–85, 87–98], and orally at 2.0 g 3 times daily in 3 trials [42, 48, 65]. In one study [86], Abelmoschus alcohol extract was given orally at 0.4 g, 3 times daily. A range of RAS blockers were used: in 13 (18.06%) studies [27, 28, 36, 44, 48, 49, 68, 69, 71, 73, 75, 86, 96] ACEI (Captopril, Enalapril Maleate, Fosinopril, and Benazepril) was used; in 52 (72.22%) studies [29–31, 33–35, 37–42, 45–47, 50, 51, 53–59, 61, 62, 64–67, 70, 76–85, 87–95, 97, 98] ARB (Valsartan, Telmisartan, Candesartan, Irbesartan, and Losartan) was used; and in 7 (9.72%) studies [32, 43, 52, 60, 63, 72, 74] either ACEI or ARB was used. All other concomitant therapies were comparable between study groups. Trials were all single-centered studies published from 1995 to 2017 and were conducted in China and published in Chinese.
Table 1.
Characteristics of 72 included studies on Abelmoschus manihot for diabetic nephropathy.
| Included studies | No. of patients | Mean age, y | Male, % | Baseline 24h UP (g/d) | Intervention | Duration (weeks) | Outcome measures | |
|---|---|---|---|---|---|---|---|---|
| Treatment group | Control group | |||||||
| Chang LL2009[27] | 128 | 47.2 | 61 | 3.7 | Benazepril 10 mg/d + HK 3.0 g tid |
Benazepril 10 mg/d | 4 | A |
| Chen XB 2014[28] | 150 | 58.4 | 51 | 2.4 | Benazepril 10 mg/d + HK 2.5 g tid |
Benazepril 10 mg/d | 8 | AFG |
| Cheng Y 2016[29] | 90 | 58.8 | 52 | NA | Valsartan 80 mg/d + HK 2.5 g tid |
Valsartan 80 mg/d | 8 | CEFG |
| Dai X 2017[30] | 80 | 46.7 | 46 | NA | Valsartan 80 mg/d + HK 2.5 g tid |
Valsartan 80 mg/d | 12 | CFG |
| Deng SY 2014[31] | 60 | 43.8 | 50 | NA | Valsartan 80 mg/d + HK 2.5 g tid |
Valsartan 80 mg/d | 16 | CFG |
| Du Y 2015[32] | 73 | 59.4 | 45 | 1.0 | ACEI/ARB + HK 2.5 g tid |
ACEI/ARB | 12 | ADFG |
| Fan HW 2014[33] | 52 | 49.1 | 48 | NA | Candesartan 4 mg/d + HK 2.5 g tid |
Candesartan 4 mg/d +placebo 2.5 g tid | 24 | CF |
| Gao Q 2017[34] | 80 | 53.7 | 68 | 3.3 | Irbesartan 150 mg/d + HK 2.5 g tid |
Irbesartan 150 mg/d | 16 | ABCFG |
| Gu J 2015[35] | 200 | 68.3 | 55 | 1.8 | Valsartan 80 mg/d + HK 2.5 g tid |
Valsartan 80 mg/d | 8 | ACDFG |
| Guan ZX 2008[36] | 80 | 45.5 | 54 | 1.4 | ACEI + HK 2.5 g tid |
ACEI | 8 | AG |
| Guo G 2015[37] | 136 | 42.8 | 53 | 0.3 | Valsartan 80 mg/d + HK 2.5 g tid |
Valsartan 80 mg/d | 8 | ABCFG |
| He YN 2010[38] | 80 | 44.4 | 54 | NA | Valsartan 80 mg/d + HK 2.5 g tid |
Valsartan 80 mg/d | 12 | EFG |
| Hu JP 2011[39] | 80 | NA | NA | 1.5 | Telmisartan 80 mg/d + HK 2.5 g tid |
Telmisartan 80 mg/d | 8 | AFG |
| Hu YY 2016[40] | 40 | 56.9 | 60 | 0.1 | Valsartan 80 mg/d + HK 2.5 g tid |
Valsartan 80 mg/d | 8 | ADFG |
| Huang XM 2016[41] | 82 | 43.4 | 57 | 0.4 | Valsartan 80 mg/d + HK 2.5 g tid |
Valsartan 80 mg/d | 16 | ABCF |
| Jia ZW 2015[42] | 70 | 51.4 | 64 | 2.7 | Candesartan 4 mg/d + HK 2.0 g tid |
Candesartan 4 mg/d | 4 | AFG |
| Jiang ZJ 2012[43] | 66 | 50.7 | 64 | 2.2 | ACEI/ARB + HK 2.5 g tid |
ACEI/ARB | 16 | AFD |
| Li HY 2009[44] | 80 | 52.1 | 50 | 1.2 | Fosinopril 10 mg/d + HK 2.5 g tid |
Fosinopril 10 mg/d | 8 | AFG |
| Li QH 2010[45] | 72 | 52.5 | 63 | NA | Valsartan 80 mg/d + HK 2.5 g tid |
Valsartan 80 mg/d | 8 | CG |
| Li WQ 2015[46] | 72 | 48.3 | 57 | 0.3 | Irbesartan 150 mg/d + HK 2.5 g tid |
Irbesartan 150 mg/d | 12 | ACF |
| Li XM 2017[47] | 62 | 60.9 | 54 | NA | Valsartan 80 mg/d + HK 2.5 g tid |
Valsartan 80 mg/d | 8 | CEG |
| Li YH 2016[48] | 65 | 49.3 | 53 | 0.9 | Benazepril 10 mg/d + HK 2.0 g tid |
Benazepril 10 mg/d | 16 | ACFG |
| Li YL 2007[49] | 60 | 41.5 | 54 | 1.5 | Benazepril 10 mg/d + HK 2.5 g tid |
Benazepril 10 mg/d | 8 | AFG |
| Li YL 2017[50] | 86 | 59.2 | 52 | NA | Valsartan 80 mg/d + HK 2.5 g tid |
Valsartan 80 mg/d | 8 | CEG |
| Li YT 2014[51] | 95 | 48.4 | 63 | NA | Valsartan 80 mg/d + HK 2.5 g tid |
Valsartan 80 mg/d | 6 | CFG |
| Li ZY 2014[52] | 126 | 57.4 | NA | 0.4 | ACEI/ARB + HK 2.5 g tid |
ACEI/ARB | 24 | ACF |
| Liang F 2015[53] | 100 | 44.8 | 54 | NA | Valsartan 80 mg/d + HK 2.5 g tid |
Valsartan 80 mg/d | 6 | CFG |
| Liang YP 2014[54] | 50 | 43.2 | 47 | NA | Valsartan 80 mg/d + HK 2.5 g tid |
Valsartan 80 mg/d | 8 | CG |
| Liao YY 2017[55] | 92 | 58.5 | 57 | NA | Valsartan 80 mg/d + HK 2.5 g tid |
Valsartan 80 mg/d | 8 | CG |
| Liu AY 2014[56] | 100 | 60.5 | 43 | 2.4 | Losartan Potassium 50 mg/d+HK 2.5 g tid | Losartan Potassium 50 mg/d | 12 | AFG |
| Liu H 2010[57] | 80 | NAa | 54 | 6.2 | Irbesartan + HK 2.5 g tid |
Irbesartan | 8 | A |
| Liu JF 2011[58] | 48 | NAa | 67 | 2.4 | Candesartan + HK 2.5 g tid |
Candesartan | 12 | AF |
| Lu C 2015[59] | 80 | 42.1 | 54 | NA | Valsartan 80 mg/d + HK 2.5 g tid |
Valsartan 80 mg/d | 8 | CG |
| Luan R 2012[60] | 96 | 42.3 | 59 | 3.8 | ACEI/ARB + HK 2.5 g tid |
ACEI/ARB | 12 | AFG |
| Ma F 2016[61] | 80 | 49.1 | 54 | NA | Valsartan 80 mg/d + HK 2.5 g tid |
Valsartan 80 mg/d | 6 | CFG |
| Meng Y 2017[62] | 86 | 45.9 | 56 | NA | Valsartan 80 mg/d + HK 2.5 g tid |
Valsartan 80 mg/d | 8 | CEG |
| Pan Q 2016[63] | 96 | 64.6 | 65 | 0.7 | Benazepril 10 mg/d or Valsartan 80 mg/d+ HK 2.5 g tid | Benazepril 10 mg/d or Valsartan 80 mg/d | 8 | ACFG |
| Qi MG 2016[64] | 84 | 60.9 | 51 | 0.2 | Valsartan 80 mg/d + HK 2.5 g tid |
Valsartan 80 mg/d | 12 | AF |
| Qian C 2014[65] | 120 | 51.0 | 53 | 2.4 | Irbesartan 150 mg/d + HK 2.0 g tid |
Irbesartan 150 mg/d | 16 | A |
| Qian CF 2010[66] | 59 | 51.1 | 51 | NA | Valsartan 80 mg/d + HK 2.5 g tid |
Valsartan 80 mg/d | 16 | C |
| Qian JL 2013[67] | 70 | 47.6 | 69 | NA | Candesartan 4 mg/d + HK 2.5 g tid |
Candesartan 4 mg/d | 24 | CFG |
| Qiao B 2015[68] | 60 | 52.8 | 33 | NA | Benazepril 10 mg/d + HK 2.5 g tid |
Benazepril 10 mg/d | 12 | CFG |
| Qiao Y 2015[69] | 82 | 56.9 | 63 | 2.4 | Enalapril 10 mg/d + HK 2.5 g tid |
Enalapril 10 mg/d | 8 | ADF |
| Qu XS 2013[70] | 56 | 45.3 | 55 | NA | Candesartan 4 mg/d + HK 2.5 g tid |
Candesartan 4 mg/d | 24 | CFG |
| Rao WP 2016[71] | 58 | 42.1 | 60 | 4.4 | Benazepril 10 mg/d + HK 2.5 g tid |
Benazepril 10 mg/d | 8 | AFG |
| Shen LL 2010[72] | 82 | 42.3 | 61 | NA | ACEI/ARB + HK 2.5 g tid |
ACEI/ARB | 12 | FG |
| Song XL 2012[73] | 60 | 40.7 | NA | 4.6 | Benazepril + HK 2.5 g tid |
Benazepril | 12 | AFG |
| Su JP 2009[74] | 65 | 54.2 | 55 | 1.0 | ACEI/ARB + HK 2.5 g tid |
ACEI/ARB | 24 | ABCDEFG |
| Sun XM 2012[75] | 90 | 62.3 | 63 | 3.7 | Benazepril 10 mg/d + HK 2.5 g tid |
Benazepril 10 mg/d | 12 | ADFG |
| Wang XC 2010[76] | 63 | 59.2 | 51 | 0.4 | Valsartan 80 mg/d + HK 2.5 g tid |
Valsartan 80 mg/d | 16 | ACF |
| Wu RK 2017[77] | 50 | 53.7 | 65 | NA | Valsartan 80 mg/d + HK 2.5 g tid |
Valsartan 80 mg/d | 6 | CEFG |
| Wu YH 2016[78] | 48 | 56.1 | 54 | 1.5 | Irbesartan 150-300 mg/d + HK 2.5 g tid | Irbesartan150-300 mg/d | 16 | AFG |
| Xiao ZZ 2010[79] | 65 | 58.3 | 52 | 0.3 | Valsartan 80 mg/d + HK 2.5 g tid |
Valsartan 80 mg/d | 16 | ABCF |
| Xu GH 2014[80] | 80 | 58.3 | 52 | 2.7 | Telmisartan 80 mg/d + HK 2.5 g tid |
Telmisartan 80 mg/d | 12 | ADFG |
| Xu RF 2012[81] | 90 | 58.0 | 64 | NA | Valsartan 80 mg/d + HK 2.5 g tid |
Valsartan 80 mg/d | 8 | F |
| Xu SS 2016[82] | 124 | 43.5 | 54 | NA | Valsartan 80 mg/d + HK 2.5 g tid |
Valsartan 80 mg/d | 24 | CF |
| Xu WM 2013[83] | 61 | 36.0 | 56 | NA | Irbesartan 150 mg/d + HK 2.5 g tid |
Irbesartan 150 mg/d | 4 | CFG |
| Yan QJ 2015[84] | 120 | 65.9 | 66 | NA | Valsartan 80 mg/d + HK 2.5 g tid |
Valsartan 80 mg/d | 8 | ACDF |
| Yan XP 2017[85] | 70 | 52.9 | 47 | NA | Valsartan 80 mg/d + HK 2.5 g tid |
Valsartan 80 mg/d | 10 | EG |
| Yu JY 1995[86] | 68 | 54.6 | 65 | 0.9 | Captopril+Abelmoschus alcohol extraction 0.4 g tid | Captopril | 8 | ADFG |
| Yu ZW 2011[87] | 58 | 69.0 | 45 | 1.5 | Candesartan 8 mg/d + HK 2.5 g tid |
Candesartan 8 mg/d | 24 | AFG |
| Zeng Y 2013[88] | 50 | 67.6 | 54 | 2.3 | Losartan 50 mg/d + HK 2.5 g tid |
Losartan 50 mg/d | 12 | AF |
| Zhang H 2011[89] | 58 | 58.2 | 55 | 1.7 | Valsartan 80-160 mg/d + HK 2.5 g tid |
Valsartan 80-160 mg/d | 8 | ACDEF |
| Zhang JW 2017[90] | 112 | 53.0 | 48 | NA | Valsartan 80 mg/d + HK 2.5 g tid |
Valsartan 80 mg/d | 16 | CG |
| Zhang RX 2016[91] | 80 | 51.3 | 53 | NA | Valsartan 80 mg/d + HK 2.5 g tid |
Valsartan 80 mg/d | 8 | CG |
| Zhang YS 2014[92] | 110 | 50.0 | 56 | 3.4 | Valsartan 80 mg/d + HK 2.5 g tid |
Valsartan 80 mg/d | 16 | ABCFG |
| Zhang ZY 2017[93] | 80 | 64.1 | 54 | 1.9 | Valsartan 80 mg/d + HK 2.5 g tid |
Valsartan 80 mg/d | 8 | ABFG |
| Zhao DH 2017[94] | 80 | 50.8 | 54 | NA | Valsartan 80 mg/d + HK 2.5 g tid |
Valsartan 80 mg/d | 6 | CEFG |
| Zhao Y 2015[95] | 92 | 49.2 | 55 | NA | Valsartan 80 mg/d + HK 2.5 g tid |
Valsartan 80 mg/d | 8 | CFG |
| Zhou BX 2008[96] | 97 | NA | NA | 0.97 | Benazepril 10 mg/d + HK 2.5 g tid |
Benazepril 10 mg/d | 8 | ACG |
| Zhou XJ 2016[97] | 96 | 66.1 | 59 | NA | Valsartan 80 mg/d + HK 2.5 g tid |
Valsartan 80 mg/d | 8 | CFG |
| Zhu JL 2017[98] | 84 | 59.8 | 58 | NA | Valsartan 80 mg/d + HK 2.5 g tid |
Valsartan 80 mg/d | 8 | CEFG |
Notes: HK: Huangkui Capsule, a single medicament of TCM extracted from the dry corolla of Abelmoschus manihot, acquired regulatory approval from China's State Food and Drug Administration (SFDA) for the treatment of chronic nephritis in 1999; ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; NA: not available; NAa: age range was reported, but mean age was not available; A: 24 h UP, 24-h urinary protein; B: eGFR: Estimated glomerular filtration rate; C: UAER, urinary albumin excretion rate; D: 24-h urinary protein (24 h UP) reduction rate, defined as the proportion of 24h UP decrease in protein excretion ≥50% of the baseline at the end of the study; E: normalization of urinary albumin excretion rate (UAER), defined as the proportion of UAER <20 μg/min upon study completion; F: SCr, serum creatinine; G: ADEs, adverse drug events.
3.2. Risk of Bias Assessment
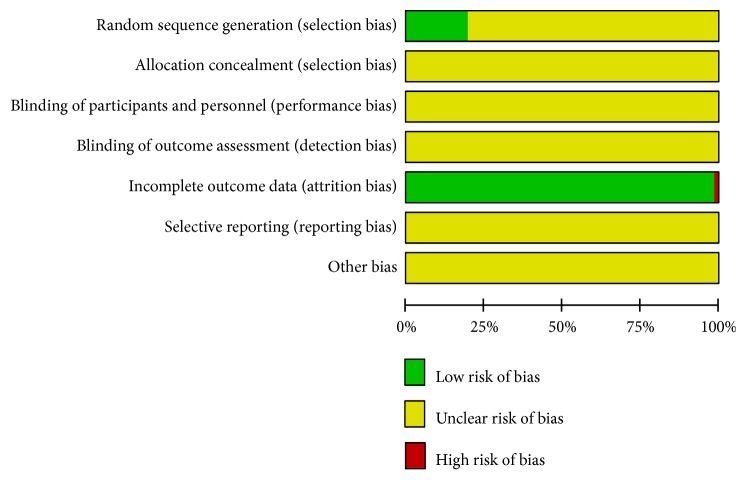
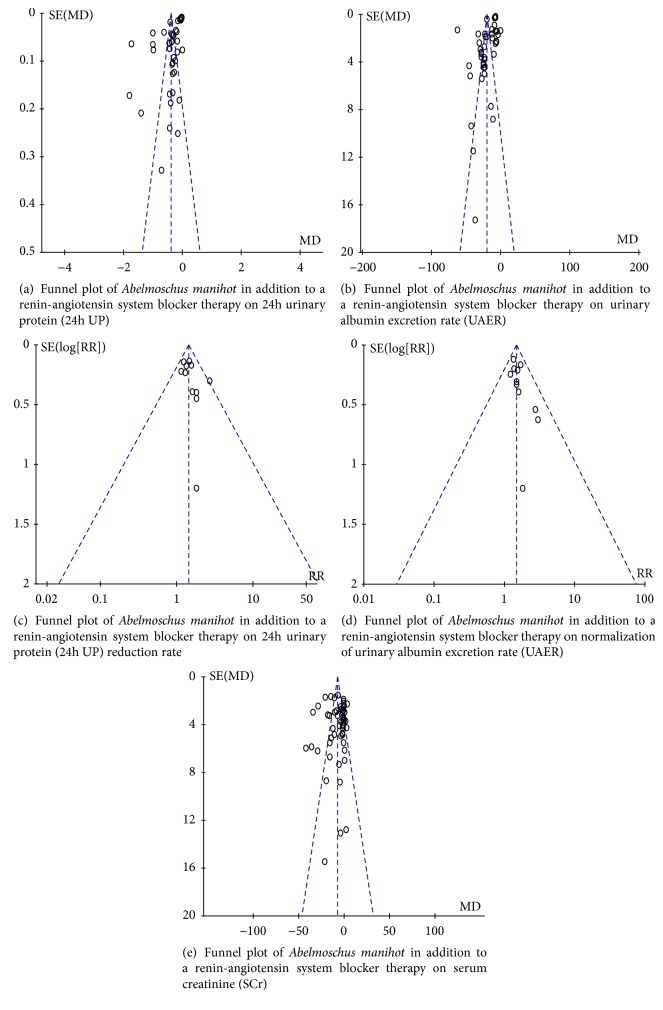
A summary of study quality is presented in Figure 2. The methodological quality was generally poor. All trials were reported to be randomized, but only 14 (19.44%) trials [28, 30, 35, 45, 47, 48, 52, 61, 65, 68, 75, 82, 88, 94] described adequate sequence generation. None of the included trials mentioned the methods for allocation concealment, the blinding of participants and personnel, and blinding of outcome assessment. Risk of attrition bias (incomplete outcome data) was detected in one [87] of all included trials, with a high risk status. Selective reporting and other potential sources of bias were unclear. Sensitivity analysis was not performed since all included trials were generally of low methodological quality. The funnel plots based on 24h UP, UAER, and SCr were asymmetrical, showing that publication bias might affect the results of this meta-analysis. The funnel plots constructed for improvements in 24h UP reduction rate and normalization of UAER were both nearly symmetrical, showing that publication bias might not affect the results of this meta-analysis. Funnel plots based on the primary and secondary outcomes are respectively elaborated in Figures 3(a), 3(b), 3(c), 3(d), and 3(e).
Figure 2.
Risk of bias graph.
Figure 3.
Funnel plots.
3.3. Effects of Interventions
3.3.1. 24-h Urinary Protein (24h UP)
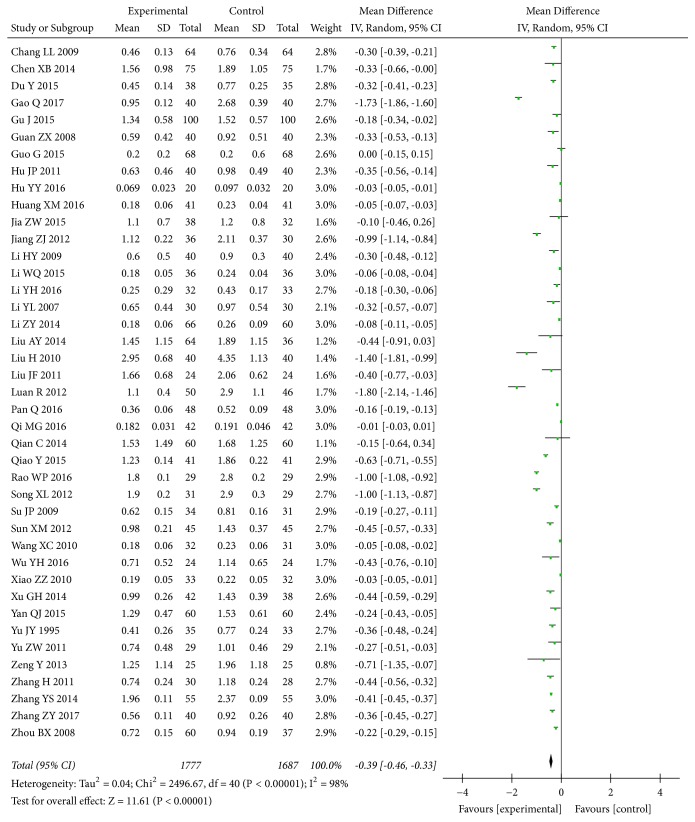
Data regarding the effect of combined A. manihot with a RAS blocker compared to a RAS blocker on 24h UP were available from 41 [27, 28, 32, 34–37, 39–44, 46, 48, 49, 52, 56–58, 60, 63–65, 69, 71, 73–76, 78–80, 84, 86–89, 92, 93, 96] of 72 trials, including 3,464 participants. The meta-analysis indicated that A. manihot plus a RAS blocker was associated with significant reductions in 24h UP level compared with a RAS blocker alone at the end of the study (MD, -0.39 [95% CI, -0.46 to -0.33] g/d; P<0.00001; Figure 4). There was evidence of significant heterogeneity across these trials (I2 =98%; P for heterogeneity <0.00001; Figure 4).
Figure 4.
Effect of Abelmoschus manihot in addition to a renin-angiotensin system blocker therapy on 24h urinary protein (24h UP).
3.3.2. End-Stage Renal Disease (ESRD) and Estimated Glomerular Filtration Rate (eGFR)
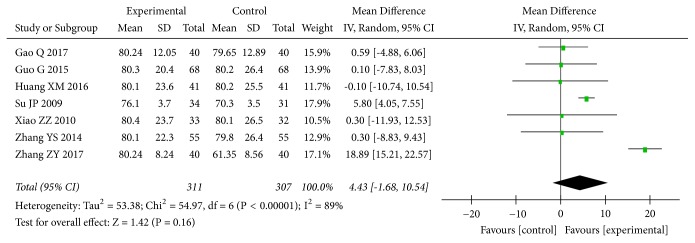
None of the included trials assessed the ESRD rate. Seven trials [34, 37, 41, 74, 79, 92, 93] with 618 patients assessed the effect of A. manihot plus a RAS blocker on eGFR in patients with DN. The results indicated that there were no statistically significant differences between A. manihot plus a RAS blocker and a RAS blocker alone in eGFR (MD, 4.43 [95% CI, -1.68 to 10.54] mL/min; P=0.16; Figure 5). There was evidence of significant heterogeneity across these trials (I2=89%; P for heterogeneity <0.00001; Figure 5).
Figure 5.
Effect of Abelmoschus manihot in addition to a renin-angiotensin system blocker therapy on estimated glomerular filtration rate (eGFR).
3.3.3. Urinary Albumin Excretion Rate (UAER)
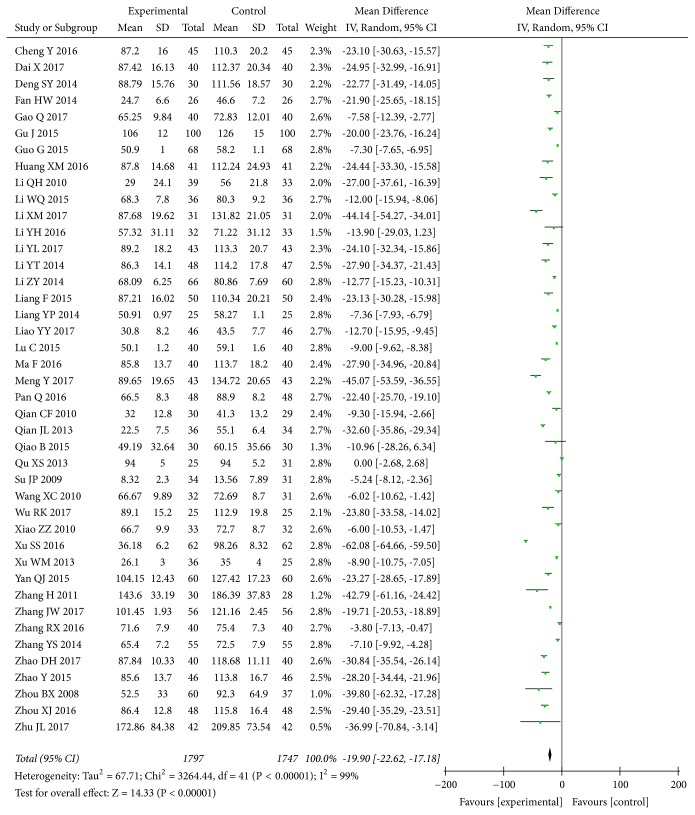
The effect of A. manihot on UAER level was reported in 42 trials [29–31, 33–35, 37, 41, 45–48, 50–55, 59, 61–63, 66–68, 70, 74, 76, 77, 79, 82–84, 89–92, 94–98], including 3,544 participants. The meta-analysis indicated that, compared to a RAS blocker alone, A. manihot combined with a RAS blocker was associated with a greater decrease in UAER (MD, -19.90 [95% CI, -22.62 to -17.18] μg/min; P<0.00001; Figure 6). Again, there was evidence of significant heterogeneity across these trials (I2=99%; P for heterogeneity <0.00001; Figure 6). In addition, two [45, 91] of 42 trials reported that trial duration per patient was 20 weeks, with 8 weeks of treatment and 12 weeks of follow-up without treatment. The MD of UAER between study groups at the end of follow-up was assessed again and still less in the treatment versus control groups (one trial [45]: MD, -33.00 [95% CI, -42.93 to -23.07] μg/min; p<0.00001, and another one [91]: MD, -11.40 [95% CI, -14.91 to -7.89] μg/min; p<0.00001), indicating that the effect of A. manihot on UAER might persist for 12 weeks after treatment.
Figure 6.
Effect of Abelmoschus manihot in addition to a renin-angiotensin system blocker therapy on urinary albumin excretion rate (UAER).
3.3.4. Improvements in 24h UP Reduction Rate and Normalization of UAER
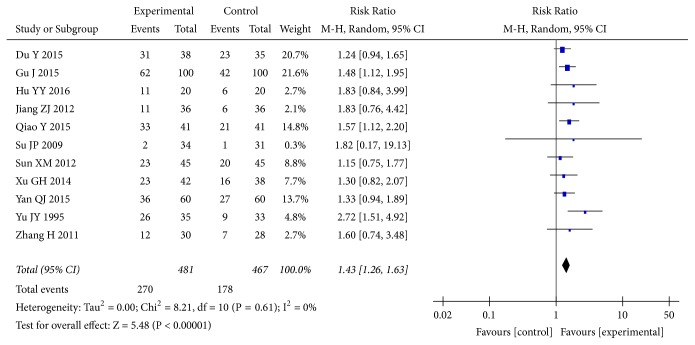
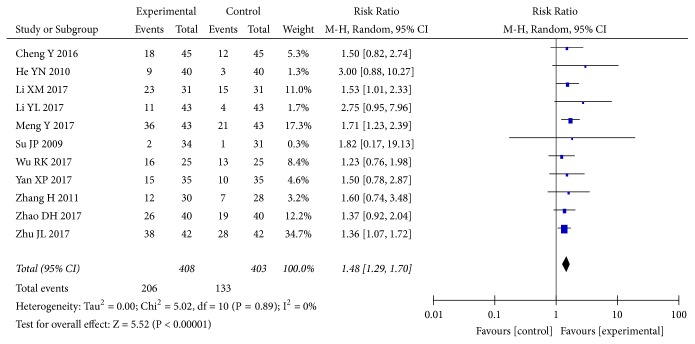
Eleven [32, 35, 40, 43, 69, 74, 75, 80, 84, 86, 89] of the included studies reported changes in 24h UP reduction rate. The pooled results showed that A. manihot combined with a RAS blocker therapy was associated with significant improvements in 24h UP reduction rate compared with a RAS blocker alone (RR, 1.43; 95% CI, 1.26-1.63; P<0.00001; Figure 7). The normalization of UAER was reported in 11 trials [29, 38, 47, 50, 62, 74, 77, 85, 89, 94, 98] of 72 RCTs. The results showed that combined treatment of A. manihot and a RAS blocker was more effective in normalization of UAER (RR, 1.48; 95% CI, 1.29-1.70; P<0.00001; Figure 8) than a RAS blocker alone. Statistical heterogeneity was low for these outcomes, suggesting a consistent effect size across studies (I2=0%; Figures 7 and 8).
Figure 7.
Effect of Abelmoschus manihot in addition to a renin-angiotensin system blocker in improving 24h urinary protein (24h UP) reduction rate. Improvements in 24h UP reduction rate, defined as the proportion of 24h UP decrease in protein excretion ≥50% of the baseline, at the end of the study.
Figure 8.
Effect of Abelmoschus manihot in addition to a renin-angiotensin system blocker in improving normalization of urinary albumin excretion rate (UAER). Normalization of UAER, defined as the proportion of UAER <20 μg/min upon study completion.
3.3.5. Serum Creatinine (SCr)
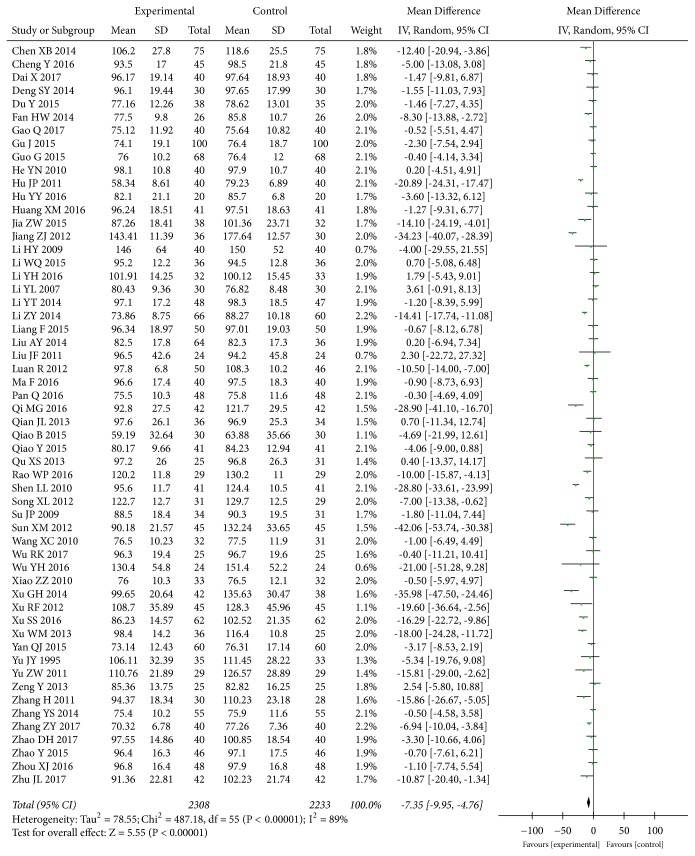
Data for the effect of A. manihot combined with a RAS blocker compared to a RAS blocker on SCr level were available from 56 trials [28–35, 37–44, 46, 48, 49, 51–53, 56, 58, 60, 61, 63, 64, 67–84, 86–89, 92–95, 97, 98] including 4,541 participants. The meta-analysis indicated that compared with a RAS blocker alone, A. manihot combined with a RAS blocker led to a greater decrease in SCr level (MD, -7.35 [95% CI, -9.95 to -4.76] μmol/L; P<0.00001, Figure 9), indicating that A. manihot was associated with improved kidney function. The I2 statistic based on the data for SCr exhibited significant heterogeneity (I2=89%, P<0.00001, Figure 9).
Figure 9.
Effect of Abelmoschus manihot in addition to a renin-angiotensin system blocker therapy on serum creatinine (SCr).
3.3.6. Adverse Drug Events (ADEs)
ADEs were observed in 53 [28–32, 34–40, 42, 44, 45, 47–51, 53–56, 59–63, 67, 68, 70–75, 77, 78, 80, 83, 85–87, 90–98] of 72 RCTs; 27 [28, 34, 36–40, 47–50, 55, 56, 62, 63, 68, 71, 73, 74, 77, 85, 86, 92–94, 96, 98] of which reported that no ADEs occurred; 26 [29–32, 35, 42, 44, 45, 51, 53, 54, 59–61, 67, 70, 72, 75, 78, 80, 83, 87, 90, 91, 95, 97] reported that ADEs occurred, including gastrointestinal discomfort, dry mouth, headache, dizziness, liver injury, hypoglycemia, hyperkalemia, coughing, and hypotension. There were no statistically significant differences between study groups in all rates of ADEs except with headache, which was reported in 10 trials [29, 35, 51, 53, 61, 75, 80, 90, 95, 97] and occurred more commonly in the control group (RR, 0.29; 95% CI, 0.11-0.76; P=0.01; I2=0%). Twenty-one trials [29–32, 35, 44, 45, 51, 53, 59–61, 67, 70, 72, 78, 83, 90, 91, 95, 97] were included in the pooled RR for gastrointestinal discomfort (RR, 1.24; 95% CI, 0.72-2.13; P=0.45; I2=0%). Eleven trials [29, 31, 35, 51, 53, 54, 59, 61, 90, 95, 97] were included in the pooled RR for dry mouth (RR, 0.51; 95% CI, 0.20-1.29; P=0.15; I2=0%). Four trials [32, 45, 75, 80] were included in the pooled RR for dizziness (RR, 0.94; 95% CI, 0.24-3.62; P=0.92; I2=0%). Four trials [42, 67, 70, 83] were included in the pooled RR for liver injury (RR, 1.40; 95% CI, 0.31-6.24; P=0.66; I2=0%). Two trials [67, 70] were included in the pooled RR for hypoglycemia (RR, 1.77; 95% CI, 0.39-8.04; P=0.46; I2=0%). One trial [87] reported three dropout cases due to hyperkalemia, of which two occurred in the treatment group and one in the control group. However, there was no statistically significant difference in the dropout rate due to hyperkalemia between study groups (RR, 2.00; 95% CI, 0.19-20.86; P=0.56). Coughing and hypotension were reported in one trial (RR, 2.84; 95% CI, 0.12-67.36; P=0.52) [67]. Nineteen [27, 33, 41, 43, 46, 52, 57, 58, 64–66, 69, 76, 79, 81, 82, 84, 88, 89] of 72 RCTs provided no data regarding ADEs despite clear descriptions of improvements in proteinuria, kidney function, and clinical symptoms. Effects of A. manihot on the likelihood of ADEs are shown in Table 2.
Table 2.
Effect of Abelmoschus manihot on the likelihood of adverse drug events.
| ADEs | No. of studies | Events | RR (95% CI) | P | |
| Treatment group (n/N) |
Control group (n/N) |
||||
|
| |||||
| Gastrointestinal discomfort | 21 | 29/912 | 20/891 | 1.24 (0.72-2.13) | 0.45 |
| Dry mouth | 11 | 5/528 | 16/527 | 0.51 (0.20-1.29) | 0.15 |
| Headache | 10 | 1/520 | 16/515 | 0.29 (0.11-0.76) | 0.01 |
| Dizziness | 4 | 4/164 | 4/151 | 0.94 (0.24-3.62) | 0.92 |
| Liver injury | 4 | 4/135 | 2/122 | 1.40 (0.31-6.24) | 0.66 |
| Hypoglycemia | 2 | 4/61 | 2/65 | 1.77 (0.39-8.04) | 0.46 |
| Hyperkalemia | 1 | 2/29 | 1/29 | 2.00 (0.19-20.86) | 0.56 |
| Coughing | 1 | 1/36 | 0/34∗ | 2.84 (0.12-67.36) | 0.52 |
| Hypotension | 1 | 1/36 | 0/34∗ | 2.84 (0.12-67.36) | 0.52 |
| Total events | 26 | 51/2421 | 61/2368 | 0.91 (0.63-1.31) | 0.61 |
Notes: ADEs: adverse drug events; CI, confidence interval; RR, risk ratio; ∗: a standard correction of 0.5 was added to all cells when a 0 cell existed in a 2X2 table for the calculation of RR.
3.4. Strength of Evidence
The GRADE approach was used to assess the quality of the evidence and risk of bias. The results are shown in Table 3. The quality of evidence was generally low.
Table 3.
GRADE Evidence Profile for Abelmoschus manihot in addition to a renin-angiotensin system blocker for diabetic nephropathy.
| Outcome | No. of studies | No. of participants | Quality assessment | Summary of findings | |||||
| Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Effect size (95% CI) | Quality | |||
|
| |||||||||
| 24h UP | 41 | 3464 | Serious1 | Serious2 | No serious indirectness | No serious imprecision | Reporting bias3 | MD, -0.39(-0.46 to -0.33) | +, Very low |
| UAER | 42 | 3544 | Serious1 | Serious2 | No serious indirectness | No serious imprecision | Reporting bias3 | MD, -19.90( -22.62 to -17.18) | +, Very low |
| 24h UP reduction rate | 11 | 948 | Serious1 | No serious inconsistency | No serious indirectness | No serious imprecision | None | RR, 1.43(1.26 to 1.63) | +++, Moderate |
| Normalization of UAER | 11 | 811 | Serious1 | No serious inconsistency | No serious indirectness | No serious imprecision | None | RR, 1.48(1.29 to 1.70) | +++, Moderate |
| SCr | 56 | 4541 | Serious1 | Serious2 | No serious indirectness | No serious imprecision | Reporting bias3 | MD, -7.35( -9.95 to -4.76) | +, Very low |
Notes: GRADE, Grades of Recommendation, Assessment, Development and Evaluation; RR, risk ratio; MD, mean difference; CI, confidence interval; 24h UP, 24-h urinary protein; UAER, urinary albumin excretion rate; SCr, serum creatinine; 1unclear allocation concealment in all studies; 2meta-analysis for the outcome exhibited significant heterogeneity; 3the funnel plot was asymmetrical, indicating a potential publication bias.
4. Discussion
4.1. Summary of Evidence
This is the first comprehensive systematic review and meta-analysis to assess the effects of A. manihot for DN patients with a diverse range of baseline protein level in urine and kidney function. None of the included trials reported the ESRD rate, and the pooled analysis of 7 trials indicated that there were no statistically significant differences between A. manihot plus a RAS blocker and a RAS blocker alone on eGFR. Thus, there was limited evidence to make a conclusion on the ESRD rate and eGFR. The results showed that compared to a RAS blocker, combined treatment of A. manihot and a RAS blocker was associated with significant improvement in proteinuria, UAER, and SCr, and the 24h UP reduction rate as well as normalization of UAER. The results also indicated that A. manihot might be generally well tolerated, because A. manihot added to a RAS blocker did not increase the rates of adverse events. However, due to the generally poor methodological quality and significant heterogeneity, there was currently insufficient evidence to support the routine use of A. manihot for DN. If confirmed in larger high-quality studies, these findings indicate that A. manihot might have an important role in improving proteinuria and protecting kidney function.
4.2. Limitations
Although this review is the most comprehensive meta-analysis to date regarding the safety and efficacy of A. manihot in combination with a RAS blocker for DN patients, there are limitations that should be considered.
Firstly, the methodological quality of these studies was generally low. Most described randomization poorly. None of the trials described allocation concealment and blinding. Only one [33] used a placebo control. One study [87] was given a grade of high risk for attrition bias (incomplete outcome data) due to the lack of information on how missing data were handled in the analysis. This meta-analysis carried the risk of reporting bias because not all studies reported all outcomes of interest. All the studies were single centered with generally small sample size, which might have resulted in the lack of power. Heterogeneity was significant among these studies, which weakened confidence in the results. Therefore, the results should be interpreted with caution due to the generally low methodological quality and significant heterogeneity.
Secondly, the study periods for all the identified studies were relatively short, resulting in the lack of evidence on the long-term effects of A. manihot for DN. In this systematic review, two studies [45, 91] reported that A. manihot was associated with a greater improvement in UAER after 8-week therapy, and the effect could persist for 12 weeks after treatment. However, most trials included assessed the short-term curative effect and did not continue with the follow-up to investigate the long-term effects of A. manihot on the prognosis of DN. Therefore, long-term studies are required to identify whether A. manihot could further reduce the rate of the ESRD.
Thirdly, close attention should be paid to ADEs. Safety is a fundamental principle for health care. Current evidence indicated that A. manihot combined with a RAS blocker might be relatively safe for DN. Nineteen of the included trials did not clearly provide data for ADEs despite all clear descriptions of great improvements in proteinuria or SCr with A. manihot therapy in this review. Future studies should pay special attention to ADEs of A. manihot.
4.3. Implication for Practice
DM is the most common cause of ESRD in the developed world [2]. In outcome trials of patients with DN, retrospective analyses demonstrate a robust relationship between magnitude of albuminuria reduction and slowing of CKD progression as well as reduced cardiovascular event rates [7–12]. The results indicated that A. manihot in addition to a RAS blocker seemed effective and safe, to reduce albuminuria further in patients with DN. However, due to the generally poor quality and significant heterogeneity, high-quality clinical studies are required to confirm these effects.
The main chemical constituents of A. manihot are flavonoids. Seven flavonoids, including hibifolin, hyperoside, myricetin, quercetin, isoquercetin, quercetin-3′-O-glucoside, and quercetin-3-O-robinobioside, were determined to be the major pharmacologically bioactive constituents of A. manihot by high-performance liquid chromatography (HPLC) [21, 99]. A. manihot was shown to improve proteinuria, renal function, kidney inflammation, and glomerular injury and attenuate renal fibrosis, podocyte apoptosis, and mesangial proliferation. The renoprotective effects of A. manihot are related to inhibition of caspase-3 and caspase-8 overexpression, reduction of the ED1+ and ED3+ macrophages, attenuation of oxidative stress (OS), downregulation of the p38 mitogen-activated protein kinase (p38MAPK) and serine-threonine kinase (Akt) pathways, the suppression of transforming growth factor-β1 (TGF-β1) and tumor necrosis factor-a (TNF-α) protein expression, as well as the inhibition of the expression of α-smooth muscle actin, phosphorylation-extracellular signal-regulated kinase (p-ERK1/2), nicotinamide adenine dinucleotide phosphate (NADPH) Oxidase 1, NADPH Oxidase 2, and NADPH Oxidase 4 [100–103].
In this analysis, the results showed that A. manihot added to a RAS blocker could further improve proteinuria and kidney function in DN patients. Four previous meta-analyses [16–19] of A. manihot for DN preliminarily reported that A. manihot therapy showed great improvements in proteinuria and kidney function, which was consistent with this analysis. The review found that A. manihot for DN was well tolerated with minimal ADEs. Since the Huangkui capsule gained national approval from the China Food and Drug Administration in 1999, there have been no reports of severe ADEs. Previous meta-analyses [16–19] of A. manihot for DN reported that the most common adverse event was mild to moderate gastrointestinal discomfort; other ADEs such as dizziness, headache, and dry mouth were rarely reported. In this analysis, nine types of adverse events were observed, including gastrointestinal discomfort, dry mouth, headache, dizziness, liver injury, hypoglycemia, hyperkalemia, coughing, and hypotension. Well-tolerated gastrointestinal discomfort was still the most common ADE. Other side effects were not frequently reported. Rates of adverse events were not significantly different between the study groups except for headache, which was reported to occur more commonly in the control group. Although 19 of the included trials provided no data for ADEs, these studies all clearly reported that A. manihot was associated with significant improvements in proteinuria, SCr, and clinical symptoms. If confirmed, these results suggest that A. manihot might be effective and relatively safe for DN.
5. Conclusions
A. manihot in addition to a RAS blocker appeared to be effective and safe to further improve proteinuria and protect kidney function in patients with DN. However, due to the generally low methodological quality, significant heterogeneity, and publication bias of included articles, high-quality clinical studies are required to confirm these findings before the routine use of A. manihot can be recommended.
Acknowledgments
We thank Bing Pang for assistance with the search strategy and methodology guidance on the GRADE approach. We would like to thank Editage [www.editage.cn] for English language editing. This work was supported by the Capital Health Research and Development of Special (no. 2016-1-4151), Beijing Natural Science Foundation (no. 7182143), and National Natural Science Foundation of China (no. 81774128).
Data Availability
All relevant data are within the paper and its supporting information files.
Disclosure
The funders did not contribute to study design, collection, analysis, interpretation of data, writing, or submission for publication.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Qing Ni conceived the study, supervised Liwei Shi to perform this review, and revised the manuscript. Liwei Shi wrote the manuscript. Qing Ni and Ling Feng arbitrated any disagreements. Liwei Shi and Meizhen Zhang developed the database search strategy and assessed studies for inclusion. Xiaowen Li and Yanan Yang extracted the data. Liwei Shi and Ling Feng performed the statistical analysis. Meizhen Zhang and Yueying Zhang assessed the quality of the evidence. Liwei Shi and Ling Feng contributed equally to this work and are co-first authors. All authors critically reviewed the report.
Supplementary Materials
File S1: PRISMA Checklist of Abelmoschus manihot for diabetic nephropathy.
File S2: PubMed search strategy.
References
- 1.Tuttle K. R., Bakris G. L., Bilous R. W., et al. Diabetic kidney disease: a report from an ADA Consensus Conference. American Journal of Kidney Diseases. 2014;64(4):510–533. doi: 10.1053/j.ajkd.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 2.de Boer I. H., Rue T. C., Hall Y. N., Heagerty P. J., Weiss N. S., Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. Journal of the American Medical Association. 2011;305(24):2532–2539. doi: 10.1001/jama.2011.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng X., Nayyar S., Wang M., et al. Mortality rates among prevalent hemodialysis patients in Beijing: a comparison with USRDS data. Nephrology Dialysis Transplantation. 2013;28(3):724–732. doi: 10.1093/ndt/gfs326. [DOI] [PubMed] [Google Scholar]
- 4.Xie Y., Chen X. Epidemiology, major outcomes, risk factors, prevention and management of chronic kidney disease in China. American Journal of Nephrology. 2007;28(1):1–7. doi: 10.1159/000108755. [DOI] [PubMed] [Google Scholar]
- 5.Fox C. S., Matsushita K., Woodward M., et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. The Lancet. 2012;380(9854):1662–1673. doi: 10.1016/s0140-6736(12)61350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tonelli M., Muntner P., Lloyd A., et al. Risk of coronary events in people with chronic kidney disease compared with those with diabetes: a population-level cohort study. The Lancet. 2012;380(9844):807–814. doi: 10.1016/s0140-6736(12)60572-8. [DOI] [PubMed] [Google Scholar]
- 7.Jafar T. H., Stark P. C., Schmid C. H., et al. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Annals of Internal Medicine. 2003;139(4):244–252. doi: 10.7326/0003-4819-139-4-200308190-00006. [DOI] [PubMed] [Google Scholar]
- 8.Schmieder R. E., Schutte R., Schumacher H., et al. Mortality and morbidity in relation to changes in albuminuria, glucose status and systolic blood pressure: an analysis of the ONTARGET and TRANSCEND studies. Diabetologia. 2014;57(10):2019–2029. doi: 10.1007/s00125-014-3330-9. [DOI] [PubMed] [Google Scholar]
- 9.Ninomiya T., Perkovic V., de Galan B. E., et al. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. Journal of the American Society of Nephrology. 2009;20(8):1813–1821. doi: 10.1681/ASN.2008121270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamout H., Lazich I., Bakris G. L. Blood pressure, hypertension, RAAS blockade, and drug therapy in diabetic kidney disease. Advances in Chronic Kidney Disease. 2014;21(3):281–286. doi: 10.1053/j.ackd.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Yakush Williams J. K. Management Strategies for Patients with Diabetic Kidney Disease and Chronic Kidney Disease in Diabetes. Nursing Clinics of North America. 2017;52(4):575–587. doi: 10.1016/j.cnur.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 12.de Zeeuw D., Remuzzi G., Parving H.-H., et al. Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation. 2004;110(8):921–927. doi: 10.1161/01.cir.0000139860.33974.28. [DOI] [PubMed] [Google Scholar]
- 13.Mavrakanas T. A., Gariani K., Martin P.-Y. Mineralocorticoid receptor blockade in addition to angiotensin converting enzyme inhibitor or angiotensin II receptor blocker treatment: An emerging paradigm in diabetic nephropathy: A systematic review. European Journal of Internal Medicine. 2014;25(2):173–176. doi: 10.1016/j.ejim.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Zhong Y., Menon M. C., Deng Y., Chen Y., He J. C. Recent advances in traditional chinese medicine for kidney disease. American Journal of Kidney Diseases. 2015;66(3):513–522. doi: 10.1053/j.ajkd.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 15.Jiang Y. R., Zhang Z. Y., Wen J., et al. Effects of okra capsule for IgA nephropathy: a systematic review. Chinese Journal of Evidence-Based Medicine. 2012;12(9):1135–1140. [Google Scholar]
- 16.Sun Q., Yang G., Zhang M., Zhang M., Chen S., Chen P. Effect of Huangshukuihua (Flos Abelmoschi Manihot) on diabetic nephropathy: a meta-analysis. Journal of Traditional Chinese Medicine. 2015;35(1):15–20. doi: 10.1016/s0254-6272(15)30003-0. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y.-Z., Gong Z.-X., Cai G.-Y., et al. Efficacy and safety of Flos Abelmoschus manihot (Malvaceae) on type 2 diabetic nephropathy: A systematic review. Chinese Journal of Integrative Medicine. 2015;21(6):464–472. doi: 10.1007/s11655-014-1891-6. [DOI] [PubMed] [Google Scholar]
- 18.Wu X. L., Li J., Liu M. Q., et al. The efficacy and safety of Huangkui capsule for the treatment of diabetic nephropathy: a systematic review. Chinese Journal of Integrated Traditional and Western Nephrology. 2014;15(12):1081–1084. [Google Scholar]
- 19.Liu H., Sun W., Gu L. B., Tu Y., Hu H. Efficacy of combination with Hangkui capsule and ACEI or ARB in treatment of diabetic nephropathy: a meta analysis. China Journal of Traditional Chinese Medicine and Pharmacy. 2015;30(5):1712–1717. [Google Scholar]
- 20.Zhang L., Li P., Xing C., et al. Efficacy and safety of Abelmoschus manihot for primary glomerular disease: a prospective, multicenter randomized controlled clinical trial. American Journal of Kidney Diseases. 2014;64(1):57–65. doi: 10.1053/j.ajkd.2014.01.431. [DOI] [PubMed] [Google Scholar]
- 21.Lai X., Liang H., Zhao Y., Wang B. Simultaneous determination of seven active flavonols in the flowers of Abelmoschus manihot by HPLC. Journal of Chromatographic Science (JCS) 2009;47(3):206–210. doi: 10.1093/chromsci/47.3.206. [DOI] [PubMed] [Google Scholar]
- 22.Chen P., Wan Y., Wang C., et al. Mechanisms and effects of Abelmoschus manihot preparations in treating chronic kidney disease. Zhongguo Zhong Yao Za Zhi. 2012;37(15):2252–2256. doi: 10.4268/cjcmm20121514. [DOI] [PubMed] [Google Scholar]
- 23.Moher D., Liberati A., Tetzlaff J., Altman D. G., The PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Open Medicine. 2009;3(3):123–130. [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins J. P. T., Altman D. G., Gøtzsche P. C., et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. British Medical Journal. 2011;343(7829) doi: 10.1136/bmj.d5928.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins J. P. T., Thompson S. G., Deeks J. J., Altman D. G. Measuring inconsistency in meta-analyses. British Medical Journal. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Egger M., Smith G. D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. British Medical Journal. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang L. L., Yang S. L., Zhao X. L., Zhang X. S., Wu W. B. The effect of Huangkui capsule on renal tubular function in patients with diabetic nephropathy. Shandong Medical Journal. 2009;49(39):56–57. [Google Scholar]
- 28.Chen X. B., Fu R. Y., Chen J. Clinical observation the effect of Benazepril combined with Huangkui capsule in the treatment of diabetic nephropathy. Journal of China Pharmacy. 2014;25(40):3764–3766. [Google Scholar]
- 29.Cheng Y., Lai C. J. Clinical curative effect observation of Huangkui capsule combined with valsartan in the treatment of diabetic nephropathy. World Chinese Medicine. 2016;11(B03):p. 1283. [Google Scholar]
- 30.Dai X., Yuan L. F., Li Y. H. Observation on clinical effect and safety on early diabetic nephropathy treated with ambrette capsule combined valsartan. Tianjin Journal of Traditional Chinese Medicine. 2017;34(3):163–164. [Google Scholar]
- 31.Deng S. Y. Clinical observation the effect of Huangkui capsule combined with Valsartan in the treatment of early diabetic nephropathy. Medical Information. 2014;27(8):p. 78. [Google Scholar]
- 32.Du Y., Li C. Q. The effect of Huangkui capsule in the treatment of diabetic nephropathy with macroalbuminuria. Chinese Journal of Integrated Traditional and Western Nephrology. 2015;16(7):610–611. [Google Scholar]
- 33.Fan H. W., Huang Y. D., Wan J. H. The effect of Huangkui capsule combined with Candesartan in the treatment of early diabetic nephropathy. China Health Care and Nutrition. 2014;24(2):p. 699. [Google Scholar]
- 34.Gao Q. Effect of Huangkui capsule combined with irbesartan on microalbuminuria and microinflammatory state in patients with diabetic nephropathy. Modern Medical Journal. 2017;45(10):1458–1462. [Google Scholar]
- 35.Gu J. The effect of valsartan combined with Huangkui capsule in the treatment of hypertension with diabetic nephropathy. Chinese Journal of Gerontology. 2015;35(23):6747–6749. [Google Scholar]
- 36.Guan Z. X., Zhang W. H. The effect of Abelmoschus manihot combined with angiotensin-converting enzyme inhibitors (ACEIs) in the treatment of diabetic nephropathy. Journal of Practical Medical Techniques. 2008;15(32):4670–4671. [Google Scholar]
- 37.Guo G. The effect of Huangkui capsule combined with valsartan in the treatment of early diabetic nephropathy. Chinese Journal of Clinical Rational Drug Use. 2015;8(7C):142–143. [Google Scholar]
- 38.He Y. N. The effect of Huangkui capsule combined with Valsartan in the treatment of 40 patients with early diabetic nephropathy. Yunnan Journal of Traditional Chinese Medicine and Materia Medica. 2010;31(6):24–25. [Google Scholar]
- 39.Hu J. P., Cao S., Luo F. The clinical effect of Huangkui Capsule combined with Telmisartan in treatment of early and metaphase diabetic nephropathy. China Journal of Chinese Medicine. 2011;26(154):353–354. [Google Scholar]
- 40.Hu Y. Y., Gao M. S., Tan Y. Effects of valsartan combined with okra capsule in treatment of early diabetic nephropathy. Nei Mongol Journal of Traditional Chinese Medicine. 2016;35(14):77–79. [Google Scholar]
- 41.Huang X. M., Zeng Q., Li H. Y. The effect of Huangkui capsule combined with valsartan in the treatment of diabetic nephropathy. Journal of Traditional Chinese Medicine University of Hunan. 2016;36(S2):p. 682. [Google Scholar]
- 42.Jia Z. W. Clinical observation the effect of Huangkui capsule combined with Candesartan in the treatment of 38 patients with early Diabetic nephropathy. Chinese Journal of Integrated Traditional and Western Nephrology. 2015;16(7):623–624. [Google Scholar]
- 43.Jiang Z. J. Clinical observation the effect of Huangkui capsule combined with Insulin Aspart 30 injection in the treatment of diabetic nephropathy. Modern Diagnosis and Treatment. 2012;23(6):755–756. [Google Scholar]
- 44.Li H. Y., Xiang F., Wang Q., Li S. F. The effect of Huangkui capsule combined with Fosinopril in the treatment of diabetic nephropathy with Mogenson stage IV. Practical Journal of Cardiac Cerebral Pneumal and Vascular Disease. 2009;17(8):691–692. [Google Scholar]
- 45.Li Q. H., He J. L. The effect of Huangkui capsule combined with Valsartan in the treatment of early diabetic nephropathy. Chinese Journal of Integrated Traditional and Western Nephrology. 2010;11(2):142–143. [Google Scholar]
- 46.Li W. Q. The effect of Huangkui capsule combined with Irbesartan in the treatment of early diabetic nephropathy. Medical Information. 2015;28(6):p. 301. [Google Scholar]
- 47.Li X. M. The effect of Huangkui capsule combined with valsartan in treatment of diabetic nephropathy. Diabetes New World. 2017;20(12):165–166. [Google Scholar]
- 48.Li Y. H., Zhang L. Y., Wang J. J., Xu Y. P., Zhang S. F. The effect of Huangkui capsule combined with Benazepril on the urinary albumin excretion rate (UAER) and C-reactive protein (CRP) in the treatment of early diabetic nephropathy. Health Guide. 2016;(49):6–7. [Google Scholar]
- 49.Li Y. L. The effect of Abelmoschus manihot combined with Benazepril Hydrochloride in the treatment of 30 patients with diabetic nephropathy. Shaanxi Journal of Traditional Chinese Medicine. 2007;28(5):562–563. [Google Scholar]
- 50.Li Y. L. Research on curative effect of clinical treatment of diabetic nephropathy. China and Foreign Medical Treatment. 2017;(11):130–132. [Google Scholar]
- 51.Li Y. T. The effect of Huangkui capsule combined with Valsartan in the treatment of diabetic nephropathy. Journal of Community Medicine. 2014;12(15):36–37. [Google Scholar]
- 52.Li Z. Y., Xie J. Clinical curative effect of Huangkui capsule for early stage diabetic nephropathy with hyperlipidemia. Chinese Journal Basic Medicine in Traditional Chinese Medicine. 2014;20(2):200–201. [Google Scholar]
- 53.Liang F., Guo J. L. Clinical observation the effect of Huangkui capsule combined with valsartan in the treatment of diabetic nephropathy. Shenzhen Journal of Integrated Traditional Chinese and Western Medicine. 2015;25(14):47–48. [Google Scholar]
- 54.Liang Y. P. The clinical curative effect of Huangkui capsule combined with Valsartan in the treatment of early diabetic nephropathy. Journal of China Prescription Drug. 2014;12(10):25–26. [Google Scholar]
- 55.Liao Y. Y. Efficacy of Huangkui capsule in the treatment of 60 patients with diabetic nephropathy. Special Health. 2017;(16):p. 207. [Google Scholar]
- 56.Liu A. Y., Feng G. Z. The effect of Losartan combined with Huangkui capsule in the treatment of diabetic nephropathy. World Health Digest. 2014;11(23):80–81. [Google Scholar]
- 57.Liu H., Zhong L. Y., Li R. H. The effect and mechanism of Huangkui capsule in the treatment of diabetic nephropathy. Chinese Journal of Integrated Traditional and Western Nephrology. 2010;11(7):633–634. [Google Scholar]
- 58.Liu J. F., Jia Y. P. Clinical observation the effect of Huangkui capsule combined with Candesartan in the treatment of 48 patients with diabetic nephropathy. Youjiang Medical Journal. 2011;39(2):202–203. [Google Scholar]
- 59.Lu C., Zhao J. X., Gou C. Y. The Effect of Huangkui Capsule Combined with Valsartan in the Treatment of Nephrotic Syndrome due to Diabetes. Perking, China: Clinical Emergency Intensive Experience Exchange Peak BBS; 2015. [Google Scholar]
- 60.Luan R., Fan S. F., Qiu B. The effect of Huangkui capsule on monocyte chemoattractant protein- 1 (MCP-1) in the treatment of diabetic nephropathy. Chinese Journal of Primary Medicine and Pharmacy. 2012;19(16):2473–2474. [Google Scholar]
- 61.Ma F., Zhao H. Y. The effect of Huangkui capsule combined with valsartan in patients with diabetic nephropathy. China Practical Medical. 2016;11(24):183–184. [Google Scholar]
- 62.Meng Y., Wang L. J., Ji X. Clinical curative effect observation of ambrette capsule combined with valsartan capsules for diabetic nephropathy. Diabetes New World. 2017;20(5):153–154. [Google Scholar]
- 63.Pan Q. Therapeutic effect of ambrette treating proteinuria in the patients with diabetes mellitus. Chinese Journal of Practical Internal Medicine. 2016;36(S2):212–213. [Google Scholar]
- 64.Qi M. G., Yu H. Y., Li R. Y. The clinical efficacy of valsartan combined with okra capsule in the treatment of early diabetic nephropathy. Inner Mongolia Medical Journal. 2016;48(3):296–298. [Google Scholar]
- 65.Qian C. The effect of Huangkui capsule combined with Irbesartan in the treatment of 60 patients with diabetic nephropathy. Chinese Journal of Ethnomedicine and Ethnopharmacy. 2014;26(20):p. 41. [Google Scholar]
- 66.Qian C. F., Qian H. The effect of Huangkui capsule combined with Valsartan on albuminuria in patients with early diabetic nephropathy. China Practical Medical. 2010;5(27):141–142. [Google Scholar]
- 67.Qian J. L. Clinical observation the effect of Huangkui capsule combined with Candesartan in the treatment of 36 patients with early diabetic nephropathy. Clinical Journal of Chinese Medicine. 2013;5(5):76–77. [Google Scholar]
- 68.Qiao B. Short-term clinical observation of early diabetic nephropathy by treating with Huang Kui capsule combined and benazepril [Master Thesis] Hebei Medical University Master's Thesis; 2015. [Google Scholar]
- 69.Qiao Y. Clinical study on the effect of Huangkui capsule combined with Enalapril Maleate in the treatment of diabetic nephropathy. Henan Traditional Chinese Medicine. 2015;35(9):2156–2158. [Google Scholar]
- 70.Qu X. S. The effect of Huangkui capsule combined with Candesartan in the treatment of early diabetic nephropathy. China Health Industry. 2013;(28):77–78. [Google Scholar]
- 71.Rao W. P. Clinical curative effect observation of Huangkui capsule combined with Benazepril in the treatment of diabetic nephropathy. The World Clinical Medicine. 2016;10(9):p. 118. [Google Scholar]
- 72.Shen L. L., Shen Y., Fang X. X., Qiu Z. L. The effect of Huangkui Capsule in the treatment of early and metaphase diabetic nephropathy. Shandong Medical Journal. 2010;50(43):59–60. [Google Scholar]
- 73.Song X. L. Clinical observation the effect of Benazepril combined with Huangkui capsule on proteinuria in the treatment of type 2 diabetic nephropathy. The Journal of Medical Theory and Practice. 2012;25(18):2238–2239. [Google Scholar]
- 74.Su J. P., Xu Y., Zhai X. L., et al. The effect of Huangkui capsule on fibrosis in the treatment of diabetic nephropathy. Chinese Journal for Clinicians. 2009;37(12):48–50. [Google Scholar]
- 75.Sun X. M., Bai J., Zhao N. Clinical observation of okra capsule and conventional therapy for diabetic nephropathy. Shanghai Journal of Traditional Chinese Medicine. 2012;46(7):54–55. [Google Scholar]
- 76.Wang X. C., Gao F. The effect of Huangkui capsule combined with Valsartan in the treatment of diabetic nephropathy. Journal of Hebei Medical University. 2010;31(6):733–734. [Google Scholar]
- 77.Wu R. K., Wang J., Xu Q. X. To explore the clinical curative effect of Huangkui capsule combined with valsartan in the treatment of diabetic nephropathy. Chinese Journal of Biochemical Pharmaceutics. 2017;37(6):236–237. [Google Scholar]
- 78.Wu Y. H. The effect of irbesartan combined with Huangkui capsule in the treatment of type 2 diabetic nephropathy. Diabetes New World. 2016;20(24):31–32. [Google Scholar]
- 79.Xiao Z. Z., Sun H. J. Effects of okra capsule combined with valsartan in treatment of early diabetic nephropathy with microalbuminuria. Modern Journal of Integrated Traditional Chinese and Western Medicine. 2010;19(3):263–264. [Google Scholar]
- 80.Xu G. H., Yuan L., Chen Y. H., et al. Clinical observation the effect of okra capsule combined with telmisartan in the treatment of early-metaphase diabetic nephropathy. Zhejiang Clinical Medical Journal. 2014;16(6):864–865. [Google Scholar]
- 81.Xu R. F. The effect of Huangkui capsule combined with valsartan in the treatment of 80 patients with diabetic kidney disease. Strait Pharmaceutical Journal. 2012;24(9):141–142. [Google Scholar]
- 82.Xu S. S., Song Z. X., Yang L. R., Yang L. The effect of Huangkui capsule combined with valsartan on Hypoxia-Inducible Factor 1(HIF-1a) and Vascular Endothelial Growth Factors (VEGF) in the treatment of diabetic nephropathy. Journal of Chinese Medicinal Materials. 2016;39(12):2885–2887. [Google Scholar]
- 83.Xu W. M., Chen H. L., Cao C. Y., Zhan H. P., Pan H. The clinical curative effect of Huangkui capsule combined with Irbesartan on oxidative stress in patients with early diabetic nephropathy. Chinese Journal of Integrated Traditional and Western Nephrology. 2013;14(10):910–911. [Google Scholar]
- 84.Yan Q. J. Clinical observation the effect of valsartan combined with Huangkui capsule in the treatment of hypertension with diabetic nephropathy. Cardiovascular Disease Journal of Integrated Traditional Chinese and Western Medicine. 2015;3(31):29–30. [Google Scholar]
- 85.Yan X. P. The effect of Huangkui capsule combined with valsartan in the treatment of diabetic nephropathy. World Latest Medicine Information. 2017;17(53):p. 150. [Google Scholar]
- 86.Yu J. Y., Xiong N. N., Guo H. F., Deng Y. Clinical observation on diabetic nephropathy treated with alcohol extraction of Abelmoschus Manihot. Journal of Integrated Traditional and Western Medicine. 1995;15(5):263–265. [PubMed] [Google Scholar]
- 87.Yu Z. W., Wang J. Z., Zhang L. P., et al. Clinical observation the effect of Huangkui capsule combined with Candesartan Cilexetil in the treatment of type 2 diabetic nephropathy. Journal of Practical Diabetology. 2011;7(3):41–42. [Google Scholar]
- 88.Zeng Y. Clinical studies on effects of combination therapy with Losartan and ambrette capsule on diabetic nephropathy. Geriatrics and Health Care. 2013;19(1):54–56. [Google Scholar]
- 89.Zhang H., Wang H. D., Du S. H., Zhang C. T. Clinical observation the effect of Huangkui capsule combined with Valsartan in the treatment of 58 patients with diabetic nephropathy. Chinese Journal of Esthetic Medicine. 2011;20(4):p. 76. [Google Scholar]
- 90.Zhang J. W. Clinical observation of Huangkui capsule combined with valsartan in the treatment of diabetic nephropathy. Journal of the Chinese Medical Association. 2017;33(23):93–94. [Google Scholar]
- 91.Zhang R. X. Clinical curative effect observation of valsartan capsules combined with ambrette capsule in treating diabetic nephropathy. Chinese Journal of Clinical Rational Drug Use. 2016;9(9A):23–24. [Google Scholar]
- 92.Zhang Y. S. The effect of Huangkui capsule combined with Valsartan on proteinuria in the treatment of diabetic nephropathy. Journal of Taishan Medical College. 2014;35(5):423–424. [Google Scholar]
- 93.Zhang Z. Y., Cao X., Chen H. Clinical curative effect observation of Huangkui capsule combined with valsartan in the treatment of diabetic nephropathy. Diabetes New World. 2017;20(3):164–165. [Google Scholar]
- 94.Zhao D. H. The clinical curative effect of Huangkui capsule combined with valsartan in the treatment of diabetic nephropathy. China Health Care and Nutrition. 2017;27(1):p. 29. [Google Scholar]
- 95.Zhao Y. The effect of Huangkui capsule combined with valsartan in the treatment of diabetic nephropathy. Medical Information. 2015;28(13):p. 45. [Google Scholar]
- 96.Zhou B. X., Bai X. M., Li D. J. Clinical curative effect observation of Huangkui capsule combined with Benazepril in treating early diabetic nephropathy. World Health Digest Medical Periodical. 2008;5(2):142–143. [Google Scholar]
- 97.Zhou X. J., Jing H. Z., Nie M. M. Clinical observation the effect of Huangkui capsule combined with valsartan in the treatment of diabetic nephropathy. China Continuing Medical Education. 2016;8(12):180–182. [Google Scholar]
- 98.Zhu J. L. The effect of Huangkui capsule combined with valsartan on the kidney function and Inflammation Mediators in patients with diabetic nephropathy. Modern Journal of Integrated Traditional Chinese and Western Medicine. 2017;26(17):1925–1926. [Google Scholar]
- 99.Chen Y., Cai G., Sun X., Chen X. Treatment of chronic kidney disease using a traditional Chinese medicine, Flos Abelmoschus manihot (Linnaeus) Medicus (Malvaceae) Clinical and Experimental Pharmacology and Physiology. 2016;43(2):145–148. doi: 10.1111/1440-1681.12528. [DOI] [PubMed] [Google Scholar]
- 100.Tu Y., Sun W., Wan Y.-G., et al. Huangkui capsule, an extract from Abelmoschus manihot (L.) medic, ameliorates adriamycin-induced renal inflammation and glomerular injury via inhibiting p38MAPK signaling pathway activity in rats. Journal of Ethnopharmacology. 2013;147(2):311–320. doi: 10.1016/j.jep.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 101.Mao Z.-M., Shen S.-M., Wan Y.-G., et al. Huangkui capsule attenuates renal fibrosis in diabetic nephropathy rats through regulating oxidative stress and p38MAPK/Akt pathways, compared to α-lipoic acid. Journal of Ethnopharmacology. 2015;173:256–265. doi: 10.1016/j.jep.2015.07.036. [DOI] [PubMed] [Google Scholar]
- 102.Zhou L., An X.-F., Teng S.-C., et al. Pretreatment with the total flavone glycosides of Flos Abelmoschus manihot and hyperoside prevents glomerular podocyte apoptosis in streptozotocin-induced diabetic nephropathy. Journal of Medicinal Food. 2012;15(5):461–468. doi: 10.1089/jmf.2011.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cai H.-D., Su S.-L., Qian D.-W., et al. Renal protective effect and action mechanism of Huangkui capsule and its main five flavonoids. Journal of Ethnopharmacology. 2017;206:152–159. doi: 10.1016/j.jep.2017.02.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File S1: PRISMA Checklist of Abelmoschus manihot for diabetic nephropathy.
File S2: PubMed search strategy.
Data Availability Statement
All relevant data are within the paper and its supporting information files.