Abstract
Leonurus cardiaca L. (motherwort) is a perennial herb, native to Asia and southeastern Europe, with widespread global occurrence in present days. The plant was historically used as cardiotonic and for treating gynaecological afflictions (such as amenorrhea, dysmenorrhea, menopausal anxiety, or postpartum depression). Although its use in oriental and occidental medicine is relatively well documented, the recent progress registered raises the need for an update of the Medicines Agency assessment report on Leonurus cardiaca L., herba (2010). The current study presents the progress made within the 2010-2018 timeframe regarding the potential applications and scientific evidences supporting the traditional use of motherwort, in the same time suggesting future research opportunities.
1. Introduction
Leonurus cardiaca L. (common names – motherwort in English, Echte Herzgespann – Deutsch, agripaume – French, etc) represents a perennial herb belonging to the Lamiaceae family. The plants grow up to 1 m, with the hollow aerial stalks growing from the rhizomes. The leaves are palmately lobed, being covered with stiff hairs. Flowers, grouped in 10-20 clusters in the leaf's axils of the last 10-15 knots, are pink and about 1 cm long [1]. The plant, original to Asia and southeastern Europe is now world-spread, due to its medicinal use [2–4]. The potential application in treating several cardiac disorders, as well as female-specific afflictions, made L. cardiaca a very good candidate for development of alternative treatments, in both traditional eastern and modern medicine [2, 5]. Besides the traditional medical use, motherwort is used in some cuisines as condiment in various vegetable soup recipes, particularly the lentil or split peas ones, or for flavoring of beer and tea [6], thus increasing the potential intake of the medicinal plant by the general public.
The current review intends to present the main findings regarding the composition and main biological activities of L. cardiaca, as emerging from the scientific studies published within the 2010-2018 timeframe. The time period was selected in order to complete the very comprehensive “Assessment report on Leonurus cardiaca L., herba” published by the European Medicine Agency [5] with the latest findings. The search methodology involved accessing and evaluating the papers found in the PubMed, ScienceDirect, Wiley Online Library, ACS Publications, and SpringerLink databases (search term “Leonurus cardiaca”, 27.12.2018). After the removal of duplicate entries, 283 studies were taken into consideration. Figure 1 describes the distribution of the reviewed works by publication year and type of paper. Most of the information presented in the present work was collected from the “Article” type papers (176 works).
Figure 1.
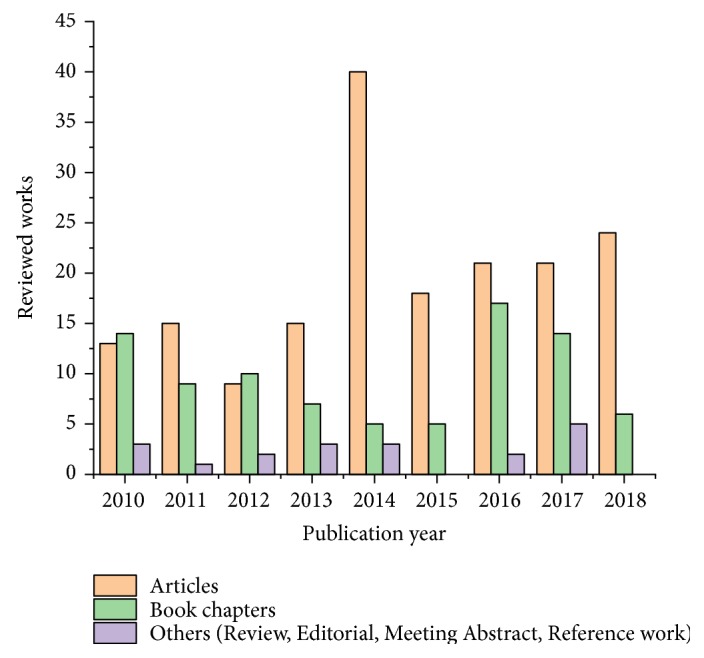
Works published in the time period 2010-2018 including L. cardiaca.
2. Composition of L. cardiaca
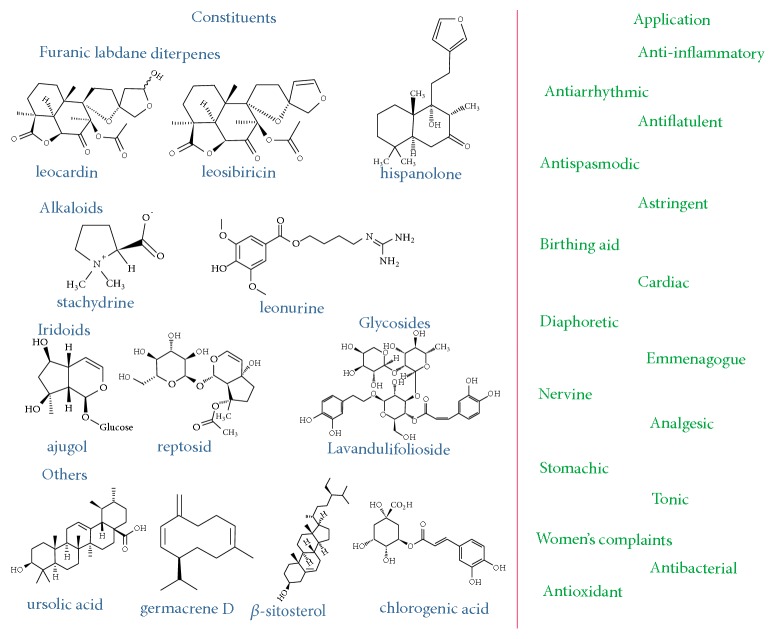
The composition of L. cardiaca was previously presented by the EMA report [5], consisting of furanic diterpenes (labdanes), alkaloids (of special interest being stachydrine), sterols, iridoids, flavonoids, ursolic acid, minerals, and others. Figure 2 presents the constituents of L. cardiaca and their potential biomedical application, as presented by the pre-2010 literature sources.
Figure 2.
Composition and applications of L. cardiaca as emerging from pre-2010 works (adapted from [5]).
The data briefly presented in Figure 2 can be completed with the findings from the time period covered by the present review. Rusch et al. [7] identified in the L. cardiaca extract the presence of a chlorinated major iridoid glucoside (7-chloro-6-desoxy-harpagide), confirmed by ESI-MS and 1d/2d 1H/13C NMR. Kuchta et al. [8] quantified by RP-HPLC the presence of ferulic acid, chlorogenic acid, caffeic acid, cichoric acid, rutoside, lavandulifolioside, verbascoside, and isoquercitrin in L. cardiaca extract, as well as stachydrine in different parts of L. cardiaca, included, for the first time in literature, in the fruits (0.2%) [9, 10].
The leaves essential oil was found to contain caryophyllene, 39.8%; α-humulene, 34.8%; α-pinene, 5.6%; β-pinene, 0.5%; linalool, 0.7%; and limonene, 0.4%, while the ursolic acid present in the leaves was quantified to be 0.26% (dry wt.) [11].
Using HPLC-MS, Zhogova et al. [12] quantified several active compounds in both medicinal plant raw material and medicinal preparation (harpagide, ajugol, galiridoside, harpagide acetate, ajugoside, galiridoside, chlorogenic acid, lavandulifolioside, verbascoside, rutin, hyperoside, isoquercitrin, and apigenin-7-O-glucoside).
An optimized recipe for the extraction of polysaccharides from motherwort leaves was presented by Tahmouzi and Ghodsi [13], obtaining a yield of 9.17 ± 0.39% for 81.4°C extraction temperature, 106.6 min. extraction time, and 45.2 ratio of water to raw material. Although the presence of leonurine in the L. cardiaca extracts is presented by some authors, including the previously cited report [5, 14], others support the contrary, not identifying the compound in the L. cardiaca extract [15].
3. Biological Activities of L. cardiaca
The main biological activities of L. cardiaca can be divided in several main categories.
3.1. Cardiovascular Action
The application of L. cardiaca in cardiovascular disorders represents one of the main applications of motherwort products [2]. Ritter et al. [16] evaluated the cardiac and electrophysiological effects of several types of L. cardiaca extracts by epicardial potential mapping and by evaluation of the effect on the cardiac ion currents using different types of cell models. The obtained results suggested that L. cardiaca extract acts as a mixed ICa.L- (L-type calcium current), IKr- (rapid delayed rectifier current) antagonist, and If (funny current, recorded in sinoatrial node cells from guinea pigs) modulator, supporting its application as an antianginal and antiarrhythmic agent.
The cardioprotective potential of ursolic acid (the natural pentacyclic triterpenoid carboxylic acid commonly found in different L. cardiaca formulations) was demonstrated by Liobikas et al. [17]. Their study revealed that the ursolic acid induced uncoupling of oxidative phosphorylation in the heart mitochondria without affecting State 3 respiration rate, in the same time suppressing the H2O2 production in isolated mitochondria, in a dose-dependent manner. The uncoupling of mitochondrial oxidation from phosphorylation, partial inhibition of the mitochondrial respiratory chain, and a reduction in the generation of free radicals in mitochondria were also observed by Bernatoniene et al. [18] in rat heart mitochondria using L. cardiaca ethanol extracts.
The clinical trial conducted by Shikov et al. [19] on fifty patients treated with 1200 mg L. cardiaca oil extract per day for 28 days revealed significant changes in systolic blood pressure, diastolic blood pressure, heart rate, and ECG for patients with stages 1 and 2 arterial hypertensions, accompanied by an improvement of psychoemotional status (anxiety, emotional liability, headache, and sleep disorders), especially visible for stage 1 patients.
Stachydrine (alkaloid found in L. cardiaca) was proven by Xie et al. [20] to ameliorate homocysteine- (Hcy-) induced endothelial dysfunction via nuclear factor erythroid 2–related factor 2 (Nrf2) dependent upregulation of guanosine triphosphate cyclohydrolase I (GTPCH1) and dihydrofolate reductase (DHFR) enzymes and increase in bioavailabilities of tetrahydrobiopterin (BH4) and nitric oxide (NO), thus protecting endothelial function.
The cardiotonic potential of L. cardiaca has been mentioned by Goetz [21, 22], Zaurov et al. [23], Brenyo and Aktas [24], Kidd [25], Jarić et al. [26], Suroowan and Mahomoodally [27], Wang et al. [28], Madridejos Mora [29], Yarnell [30], Orhan et al. [31], Dong et al. [2], and Bianchi [32].
3.2. Anti-Inflammatory, Antimicrobial Effect, and Application in Female Disorders
The anti-inflammatory potential of leonurine (considered by some authors a natural component of L. cardiaca [5]) on E. coli-induced mastitis in mice [33]: their results suggested that leonurine alleviates the histopathological changes, downregulates the levels of proinflammatory cytokines (TNF-α and IL-6), upregulates the level of anti-inflammatory cytokine IL-10, and inhibits the expression of nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2). The suggested mechanism involves the inhibition of expression of toll-like receptor 4 (TLR4) and nuclear factor-kappa B (NF-κB) activation and mitogen-activated protein kinases (p38), extracellular signal-regulated kinase (ERK), and Jun N-terminal kinase (JNK) phosphorylation. The anti-inflammatory potential of leonurine was also demonstrated by Liu et al. [34] in rat animal models of acute gouty arthritis. The obtained results support the use of leonurine as COX-2, mPGES-1 (microsomal prostaglandin E synthase-1), and 5-LOX (5-lipoxygenase) inhibitor, leading to antiarthritis effects. In the same time, amelioration of monosodium urate crystal-induced inflammation was achieved by decreasing interleukin-1β (IL-1β) and tumor necrosis factor-alpha (TNF-α) production.
The immunomodulatory potential of acetone/water extract of L. cardiaca at a 100 µg/ml concentration was assessed by Sadowska et al. [35], revealing a significant reduction of the platelet aggregation in the presence of arachidonic acid, an application that could be beneficial in preventing inflammatory lesions. In the same time, the tested extract did not exhibit proapoptotic activity.
The traditional use of L. cardiaca as an anti-inflammatory and antimicrobial agent was also supported by the results presented by Flemmig et al. [36]. They tested several extracts and components of L. cardiaca for their ability to regenerate the pseudo-halogenating activity of lactoperoxidase (LPO). The results supported the use of components with a 3,4- dihydroxyphenylic partial structure (such as caffeic acid derivatives or phenylethanoids) as efficient LPO activity regenerators, as well as the use of L. cardiaca ethanol extract for the same application. The same study also presents the isolation of the compound caffeoylmalic acid, which also revealed moderate LPO activity-regenerating effects.
Micota et al. [37] studied the antimicrobial potential of L. cardiaca acetone-water extract and its component ursolic acid by determining the minimal inhibitory concentration, as well as the antiadhesive and antibiofilm properties against Staphylococcus aureus strain (potential etiological agent of infective endocarditis). Their results (MIC = 6 mg/ml for extract and 0.25 mg/ml for ursolic acid) showed weak biostatic activity of L. cardiaca extract in comparison to ursolic acid, but both preparations possessed antiadhesive potential. The S. aureus biofilm formation was slightly inhibited by the extract (5%), but strongly inhibited by ursolic acid (85%) at concentrations of 3/4 MIC.
The study of Samoilova et al. [38] on the effect of subinhibitory doses of plant extract (including L. cardiaca) on Escherichia coli biofilm formation revealed that motherwort extract showed a synergistic effect with sublethal concentration of streptomycin (30 µg/ml), inhibiting the specific biofilm formation. As the study was focused on the capacity of low concentration plant extracts and polyphenols to induce adaptive mechanisms in E. coli, it cannot be considered a truly antimicrobial study.
Micota et al. [39] used subinhibitory doses of L. cardiaca extract to establish its effects on the characteristics of Staphylococcus aureus. The beneficial effect of the extract was observed, such as reduction in staphylococcal adherence, aggregates formation, coagulase activity, protein A expression, or alpha-toxin synthesis. However, some of their findings (e.g., enhancement of staphylococcal tolerance to exogenous hydrogen peroxide after preincubation with the extract) led to the conclusion of a possible risk of adverse effects.
Wu et al. [40] presented the application of leonurine for ameliorating the inflammatory responses in endometritis model in mice. The leonurine treatment suppressed the TNF-α and IL-1β mRNA levels in uterus tissues, inhibited lipopolysaccharide-induced TLR4 expression, and reduced the phosphorylated p65 and IκBα proteins.
The clinical trial conducted by Denham et al. [41] documented the herbal prescribing in usual practice, covering a total of 80 herbs on 141 prescriptions (the most encountered being L. cardiaca - 77%) for treatment of symptoms associated with the menopause on 35 subjects. L. cardiaca was mainly prescribed to control hot flushes, as a gynecological tonic and as a relaxant.
In a randomized study conducted on 165 women undergoing cesarean section, motherwort, in combination with oxytocin, proved to be efficient for preventing postpartum hemorrhage [42].
Regarding the use of L. cardiaca in female disorders, the plant is listed as a natural remedy for female reproductive system (anxiolytic, antispasmodic, PMS, and menopausal anxiety) [43], as an emmenagogue, nervine, amenorrhea, analgesic, and uterine astringents/vascular decongestants and for treating adolescent dysmenorrhea [44], for treating menopausal anxiety, and as tranquilizer [45]. Lans et al. [46] present motherwort as a natural cure used in North America from colonial times, due to its tonic, emmenagogue, antispasmodic, and nervine properties, citing pre-1900 works.
3.3. Antioxidant Action
The antioxidant activity of L. cardiaca products was evaluated using several methods. Sadowska et al. [35] evaluated the antioxidant potential of L. cardiaca extract by ABTS∙, DPPH∙, and ferric reducing antioxidant power assay, obtaining values of the antioxidant capacity in the range 350±20–455±17 µM Trolox/g. Ebrahimzadeh et al. [47] evaluated the antioxidant potential of Iranian native L. cardiaca extract obtained from dried aerial parts by percolation using methanol, by comparison with Grammosciadium platycarpum and Onosma demawendicum extracts. The results obtained by DPPH∙ assay (IC50 = 144 ± 12.1 mg/ml), iron reducing assay (results superior to vitamin C in the concentration range 25-100 µg/ml), nitric oxide-scavenging assay (IC50 = 0.15 ± 0.01 mg/ml), metal chelating assay (IC50 = 20 ± 1 µg/ml), and scavenging of hydrogen peroxide (IC50 = 438.2 ± 21.8 µg/ml) were correlated with the total phenol content (54.3 ± 1.8 mg gallic acid equivalent/g of extract). Ebrahimzadeh et al. [48] evaluated the correlation between the total phenolic compounds and total flavonoids content and the nitric oxide scavenging properties for 26 Iranian medicinal plants. The authors found good correlation between total phenolic content (54.3 ± 2.71 mg gallic acid equivalent/g of extract) for L. cardiaca aerial parts methanol extract, total flavonoids content (35.2 ± 1.76 mg quercetin equivalents/g of extract), and nitric oxide radical scavenging activity (IC50 = 0.15 ± 0.007 mg/ml).
Armatu et al. [49] evaluated the antioxidant potential of several extracts obtained from Romanian Lamiaceae species (including L. cardiaca methanol extract) using the DPPH∙ assay, phosphomolybdenum method, and chemiluminescence assay in relationship with HPTLC fingerprints and total phenolic content. The results obtained by the three antioxidant assays at 5 mg/ml concentration (DPPH∙ – 20%, total antioxidant capacity –approx. 40 mg ascorbic acid equivalents/g and 48% antioxidant activity for the chemiluminescence activity) were correlated with the relatively low total phenolics content (2.8 mg gallic acid equivalents/g of extract).
Jafari et al. [50] evaluated the total phenolic content and antioxidant capacity (DPPH∙ assay) of different fractions of Iranian L. cardiaca extract. The best results were obtained for the 50:50 metanolic-aqueous fraction (total phenolic content 70.79±4.41 gallic acid equivalents/g of fraction and IC50 = 53.79 µg/ml – DPPH∙ assay).
The influence of drying method was studied by Yi and Wetzstein [51] using three drying methods (greenhouse sun-drying, 40°C oven-drying, and 70°C oven-drying) on 80% methanol and 80% ethanol extracts from the leaves of cultivated L. cardiaca plants. The best results were obtained for ethanol extracts of 40°C oven-dried plants (total polyphenolics – approx. 70 mg/g GAE, Trolox-equivalent antioxidant capacity – approx. 400 mM/g TE).
Polysaccharides from L. cardiaca extract exhibited a very good scavenging activity of hydroxyl radicals (IC50 = 6.98 ± 0.87 mg/mL), compared with vitamin C (IC50 = 7.59 ± 0.94 mg/mL) [13]. Wong et al. [52] evaluated the antioxidant potential and phytochemical composition of aqueous extracts obtained from Malaysian plants. The extract showed a relatively good antioxidant potential (>60% DPPH∙ scavenging activity at 16 mg/ml, >80% NO scavenging activity at 10 mg/ml, and >85% metal chelating activity at 10 mg/ml) for a phytochemical composition of 12.13 ± 0.12 mg GAE/g dry weight (total phenolics), 9.86 ± 0.15 mg QE/g dw (total flavonoids), and 2.01 ± 0.01 mg CAE/g dw (hydroxycinnamic acids). The study of Ji et al. [53] on the antioxidant effect of plants with therapeutic potential on gynecological diseases revealed no significant influence on the lag phase duration of copper-induced low-density lipoprotein cholesterol (LDL-C) oxidation, the authors suggesting as main reason for the lack of antioxidant activity the solvent used for extraction. Ziyatdinova et al. [54] proposed an alternative method for the evaluation of the antioxidant activity (determined as DPPH∙ inhibition using differential pulse voltammetry) and compared the values obtained for several medicinal plants with those obtained spectrophotometrically. The results showed better antioxidant effect for the L. cardiaca infusion (32±1%, determined by differential pulse voltammetry, respectively, 33±1%, determined by spectrophotometry) compared with the tincture (70% ethanol, 16.6±0.4%, determined by differential pulse voltammetry, respectively, 17±2% determined by spectrophotometry). Ziyatdinova et al. [55] described a chronocoulometric method for the evaluation of the antioxidant potential of 42 commercial-available medicinal plants tinctures (including L. cardiaca), also establishing a correlation between the antioxidant potential and the total phenolics content. The evaluation revealed a relatively low antioxidant capacity for the L. cardiaca tincture (1.7±0.02 mg quercetin/mL).
The antioxidant potential of L. cardiaca extracts was also briefly presented in other works (for example, the works of Sen and Chakraborty [56] and Krishnaiah et al. [57]).
3.4. Other Applications
Motherwort was historically used for the treatment of several nervous afflictions, such as depression, anxiety, or stress [57]. Romm [58] classified L. cardiaca as a natural remedy for the treatment of several afflictions, including postpartum depression, while the traditional internal use of motherwort for the treatment of epilepsy was documented by Adams et al. [59]. The potential towards the treatment of anxiety and depressive disorders was evaluated by Rauwald et al. [15, 60], by studying the effect of L. cardiaca extract and constituents (isoleosibirin, 7R-chloro-6-desoxy-harpagide, lavandulifolioside, stachydrine, and leonurine) on the neuronal receptor gamma-aminobutyric acid (GABA). The extract inhibited the concentration-dependent binding of [(3)H]-SR95 531 to the GABA site of the GABA type A receptor with a binding affinity (IC50) of 21 µg/ml, suggesting a potential neurological mechanism of action of L. cardiaca, based on interaction to the GABA site of the GABA type A receptor. The individual components tested (except leonurine – IC50 - 15 µg/ml) did not exhibit significant activity. Commercially available leonurine was demonstrated by Xu et al. [61] to ameliorate LPS-induced acute kidney injury in mice. The nephroprotective effect was expressed, after 14 days of treatment, by the values of reactive oxygen species (ROS), malonyldialdehyde (MDA), and reduced glutathione (GSH) which were reduced to near control levels, while the lipopolysaccharide-induced tubular damage was significantly ameliorated, decreased renal injury biomarker (KMI-1), and inhibited the nuclear transfer of NF-κB p65. A similar nephroprotective effect was registered by Cheng et al. [62] in mouse unilateral urethral obstruction, by suppressing ROS-mediated TGF-β/Smad3-induced tubulointerstitial fibrosis and inhibiting NF-κB-mediated inflammatory response. Leonurine was also found to ameliorate cognitive disfunction in rats' model [63]. At a 100 concentration, it was found to decrease the oxygen-glucose deprivation- (OGD-) induced brain cell death to approx. 130% (fold of control group), an approx. 50% reduction, compared with the OGD group. Also, leonurine was found to alleviate the impaired spatial learning and memory, as demonstrated through Morris water maze test. Leonurine also decreased the concentrations of glutamate and hydrogen peroxide in hippocampus, ameliorated the impaired long-term depression in hippocampus, improved cognitive function by modulating the N-methyl-D-aspartate receptors-associated proteins, and protected rats from bilateral carotid artery occlusion-induced damage by inhibiting autophagy. The results suggested leonurine as a potential drug candidate for chronic cerebral hypoperfusion.
Ethanolic extract obtained from aerial parts of L. cardiaca was evaluated by Rezaee-Asl et al. [64] as a potential analgesic, using formalin, tail flick, and hot plate tests in mice. The results of the study proved that at 500 mg/kg the extract was able to reduce the formalin-induced pain in the early phase, increase the antinociceptive activity, and significantly influence the reaction time of the animals to the hot plates, supporting the analgesic properties of the extract, action mediated through peripheral, and central inhibitory mechanisms.
The antiviral potential of L. cardiaca was reviewed by Todorov et al. [65]. Different components of the extract were found to be active against several types of viruses (ursolic acid - HCV, HPV-18; quearcetin - HSV-1, poliovirus 1, RSV; hyperoside – DHBV; apigenin – HSV-2; rutin – HIV-1), while the chloroform and methanol extracts were presented to possess antiherpes activity against HSV-1 and 2.
Onumah [66] presents L. cardiaca as a potential adjuvant in treating overactive thyroid, due to its action against symptoms associated with hyperthyroidism (palpitations and anxiety). Inhibition of the thyroid-stimulating hormone and the reduction of excess production of thyroid hormones were also presented by Shokri et al. [67], the property being assigned to its content in rosmarinic acid.
Unlike many other plant species, the evaluated literature data (even outside the time period covered by the present review) presents no research regarding the phytosynthesis of metallic nanoparticles using L. cardiaca extracts, although the procedure was presented for L. japonicus [68].
Table 1 summarizes the main biological activities presented.
Table 1.
Biological activities of L. cardiaca (2010-2018).
| Origin | Part of plant/product | Type of paper | Activity | Tests performed | Components responsible for activity | Ref. |
|---|---|---|---|---|---|---|
| - | Aerial parts | Review | Against uterine infection or other gynecological diseases, tachyarrhythmia and other cardiac disorders | - | - | [2] |
| - | Aerial parts | Review | Antioxidant | - | Rutin and derivatives of hydroxycinnamic acid | [2] |
| Commercially available, Germany | Aerial parts extract (aqueous Soxhlet) and refined extracts: dichloromethane, 70% hydroethanolic | Research | Antianginal and antiarrhythmic | Epicardial potential mapping, effect on the cardiac ion currents | phenolic constituents | [16] |
| Commercially available | Ursolic acid | Research | Cardioprotective | Evaluation of mitochondrial respiratory rates, mitochondrial H2O2 generation, H2O2 antioxidant activity | - | [17] |
| Wild-growing, Lithuania | 70% ethanol extract | Research | Cardioprotective | Measurement of the mitochondrial respiration rate and mitochondrial H2O2 generation | Chlorogenic acid and flavonoids orientin, quercetin, hyperoside, and rutin |
[18] |
| - | Soybean oil extract | Clinical trial | Treatment of arterial hypertension accompanied by anxiety and sleep disorders | dynamics of psychoneurological symptoms; state – activity – mood, the Clinical Global Impression scale, systolic blood pressure, diastolic blood pressure, heart rate and ECG |
Iridoids | [19] |
| Commercially available | Stachydrine | Research | endothelial function protection | Determination of cell viability, Nitric oxide assay, Measurement of BH4, Measurement of cGMP levels in rat arterial rings. Measurement of vasorelaxation, Quantitative reverse transcriptase-PCR (qRT-PCR), Western blotting | - | [20] |
| - | Extract | Review | Sedative, cardiotonic | - | [21] | |
| - | Aerial parts extract | Review | cardiotonic bradycardic agent, hypotensive | - | - | [22] |
| - | Aerial parts tincture | Review | Sedative, decreases arterial pressure and strengthens the contraction of uterus muscles |
- | - | [23] |
| - | Stachydrine | Review | Protective effect in experimental myocardial ischemia reperfusion injury |
- | - | [23] |
| - | - | Review | Sedative, antispasmodic, electrophysiologic | [24] | ||
| - | Lavandulifolioside | Review | chronotropic effects | - | - | [24] |
| - | - | Review | Cardiovascular | - | - | [25] |
| - | Aerial parts, tea | Review | Strengthening the heart, arrhythmia, antihypertensive | - | - | [26] |
| - | - | Review | Cardiac arrhythmias, tachycardia, heart palpitations | - | phenylpropanoid glycosides | [27] |
| - | Aerial parts | Review | Cardioprotective, antioxidant | - | - | [28] |
| - | - | Review | relief of nervous tension symptoms, treatment of arrhythmias | - | - | [29] |
| - | - | Review | maintenance of normal cardiac rhythm | - | - | [30] |
| - | - | Review | Cardiotonic | - | - | [31] |
| - | - | Review | Sedative, vasodilator | - | - | [32] |
| Commercially available | Leonurine | Research | Anti-Inflammatory | Histopathological analysis, Cytokines Analysis, Quantitative Real-Time Polymerase Chain Reaction, Western Blot Analysis | - | [33] |
| Commercially available | Leonurine | Research | Agent for gouty arthritis treatment | Histological examination, Lentiviral transduction, Western blot analysis, Cytokine measurement | - | [34] |
| Commercially available, Poland | Acetone-water (70:30, v/v) extract | Research | Immunomodulatory, antioxidant | NO production in Human umbilical vein endothelial cells, platelet aggregation, ABTS∙, DPPH∙ and FRAP assay | - | [35] |
| Commercially available, Germany | Ethanol extract (70%), Soxhlet extraction, Methanol extract, other phases | Research | Anti-inflammatory and antimicrobial |
Effect of extracts and single components on lactoperoxidase activity |
Phenolic components with 3,4-dihydroxyphenyl partial structure |
[36] |
| Commercially available, Poland | Leaves acetone-water (70:30, v/v) extract | Research | Antimicrobial | Evaluation of antimicrobial, anti-adhesive and anti-biofilm properties against S. aureus | Iridoid glycosides, di- and triterpenoids, flavonoids, tannins and volatile oils | [37] |
| Commercially available, Russia | Aerial parts water extract | Research | Anti-biofilm formation | Biofilm formation assay | - | [38] |
| Commercially available, Poland | Aerial parts polyphenol-enriched extract | Research | Effect of sub-inhibitory concentration extracts on S. aureus | S. aureus survival, staphylococcal tolerance to oxidative stress, S. aureusα-toxin (Hla) release and protein A (SpA) expression, Staphylococcal aggregation in human plasma, Fibrinogen polymerization and S. aureusadhesion to fibrin | - | [39] |
| Commercially available | Leonurine | Research | Anti-inflammatory | Histopathological analysis, Cell viability assay, Analysis of cytokines, qRT-PCR analysis, immunoblotting analysis, | - | [40] |
| - | Aerial parts, tea | Clinical trial | To control hot flushes, gynaecological tonic, relaxant | - | - | [41] |
| - | Motherwort injection | Trial | Preventing postpartum hemorrhage | Mean blood loss, postpartum hemorrhage, mean systolic blood pressure, diastolic BP, heart rate, respiratory rate, hemoglobin and platelet count, incidence of postpartum hemorrhage, safety assessment | - | [42] |
| - | Aerial parts | Review | Anxiolytic, antispasmodic, PMS, and menopausal anxiety | - | - | [43] |
| Aerial parts, tea, tincture, infusion | Review | Antispasmodic, anti-inflammatory anxiolytic, uterine tonic, | - | - | [44] | |
| - | Aerial parts, tincture, tea, infusion | Review | Menopausal anxiety, insomnia, palpitations hyperthyroidism, | - | - | [45] |
| - | Aerial parts, infusion, decoction | Review | Tonic, Amenorrhoea, suppressed lochia, dysmenorrhoea, antispasmodic, nervine, emmenagogue | - | - | [46] |
| Wild-growing, Iran | Aerial parts, methanol extract | Research | Antioxidant, radical scavenging | DPPH radical-scavenging activity, reducing power, nitric oxide-scavenging activity, Metal chelating activity, ferric thiocyanate assay, Scavenging of hydrogen peroxide | Phenolic compounds | [47] |
| Wild-growing, Iran | Aerial parts, methanol and water extracts | Research | Antioxidant | Nitric oxide radical scavenging activity | Phenolic compounds, flavonoids | [48] |
| Cultivated, Romania | Aerial parts, methanol extract | Research | Antioxidant, free scavenging potential | phosphomolybdenum reduction assay, DPPH∙ assay, Chemiluminescence assay | Polyphenolics | [49] |
| Cultivated, Iran | Aerial parts, extracts and fractions | Research | Antioxidant | DPPH∙ assay | Phenolic compounds | [50] |
| Cultivated, Greece | Leaves, 80% methanol or 80% ethanol | Research | Antioxidant | ABTS∙ assay | Phenolic compounds | [51] |
| Wild-growing, Iran | Leaves, polysaccharides extract | Research | Antioxidant, antimicrobial | Hydroxyl radical scavenging capacity, DPPH∙ assay, antimicrobial effect evaluated by the filter disk diffusion plate method against bacteria, yeast and fungi | Polysaccharides | [13] |
| Wild-growing, Malaysia | Aerial parts, water extract | Research | Antioxidant, antidiabetic | DPPH∙ assay, nitric oxide radical scavenging assay, metal chelating activity, Alpha-glucosidase inhibition assay | Phenolic compounds | [52] |
| Commercially available, China | Aerial parts, aqueous extract | Research | Antioxidant | LDLc oxidation delay | - | [53] |
| Commercially available, Russia | Commercial tinctures and infusions | Research | Antioxidant | DPPH∙ assay, voltammetry and spectrophotometric | - | [54] |
| Commercially available, Russia | Commercial tinctures | Research | Antioxidant | Chronocoulometry | Lavandulifolioside, phenolic acids, caffeic acid 4-rutinoside, tannins | [55] |
| - | - | Review | Antioxidant | - | - | [56] |
| - | Methanol extract | Review | Antioxidant | DPPH∙ assay | Flavonoid and phenolic glycosides |
[57] |
| - | Aerial part, tincture, tea | Review | Nervine relaxant, anxiolytic, | - | - | [58] |
| - | - | Review | Epilepsy treatment | - | - | [59] |
| Commercially available, Germany | Aerial parts, 45% ethanol extract, Stachydrine, Leonurine, Lavandulifolioside, Isoleosibirin, 7R-chloro-6-desoxy-harpagide | Research | Treatment of anxiety, depression, nervousness, sedative | In vitro GABA receptor binding assays, | - | [15, 60] |
| Commercially available | Leonurine | Research | Nephroprotective | Measurement of TNF-α, IL-1, IL-6, IL-8, Measurement of serum creatinine and blood urea nitrogen, Assay of GSH, Assay of ROS, Assay of MDA level, Western blot analyses, Protein assay, Histological assay | - | [61] |
| Commercially available | Leonurine | Research | Nephroprotective | Measurement of TGF-β, TNF-α, IL-6 and IL-1β level, ROS assay, Assay of MDA level, Assay of GSH level, Western blot analyses, Protein assay, Histological assay | - | [62] |
| Commercially available | Leonurine | Research | Neuroprotective | Evaluation of spatial learning and memory performances of rats, levels of glutamate and H2O2 of hippocampus, Western blot assay | - | [63] |
| - | Aerial parts, ethanolic extract | Research | Analgesic | Nociceptive Behavioral Tests | - | [64] |
| - | Aerial parts, different types of extracts | Review | Antiviral | - | ursolic acid, quercetin, hyperoside, apigenin-7- glucoside, rutin |
[65] |
| - | - | Review | Treatment of hyperthyroidism, palpitations, anxiety | - | - | [66] |
| - | - | Review | Treatment of hyperthyroidism | - | Rosmarinic acid | [67] |
4. Dosage and Toxicology
As previously has been presented, L. cardiaca preparations are currently used in the treatment of several conditions. Relative to commercial products, the EMA report cited [5] presents them to be safe, suggesting a duration of use limited to four weeks. The report also presents the adverse effects of an intake of 3.0 grams of a powdered extract per day (diarrhea, uterine bleeding, and stomach irritation). L. cardiaca is listed as a “herb to avoid during pregnancy” [69, 70], mainly due to its emmenagogue and uterine stimulation properties. Kaye et al. [71] listed L. cardiaca as a “herbal drug associated with bleeding abnormalities”, an aspect to be considered by the anesthesia practitioner.
Anadón et al. [72] presented the reduction of platelet aggregation and fibrinogen levels upon intravenous administration of motherwort. L. cardiaca also potentiates antithrombotic and antiplatelet effects, increasing the risk of bleeding. When administered concomitant with benzodiazepines, motherwort can also have a synergistic sedative effect resulting in coma [73].
L. cardiaca was also presented to potentialize the effect of warfarin, by inhibiting platelet aggregation [74].
Related to individual compounds, data are scarce and mainly outside the current review time period: Milkowska-Leyck et al. presented moderate toxicity of lavandulifolioside (LD50 approx. 1000 mg/kg) and n-butanol extract (LD50 approx. 400 mg/kg) for intravenous administration, while for oral administration, the toxicity was much lower (LD50 >2000 mg/kg) [75]; Mitchell and Rook [76] present the potential of L. cardiaca leaves to cause photosensitization and dermatitis; herbal intravenous injection has a LD50 of 30-60 mg/kg (mice), while the intravenous LD50 of the total alkaloids of the herb was approx. 570 mg/kg, while the minimal lethal dose of leonurine in frogs (subcutaneous administration) was 400-600 mg/kg [77]. Due to the lack in scientific evidences, most of the sources presenting traditional use of motherwort suggest the strict following of relevant directions on products containing L. cardiaca and the requesting supplemental information from pharmacists, physician, or other healthcare professionals before use [78]. Some chemical components from L. cardiaca aerial parts (pyrrolidine alkaloids, such as stachydrine, cyclic peptide, such as cycloleonurinine or labdane diterpenes, such as leosibiricin) are considered “chemicals of concern” for human health when used in food and food supplements [79].
5. Conclusions
The use of motherwort (Leonurus cardiaca L.) has been documented since ancient times, especially as a cardiotonic and for the treatment of gynecological conditions. The composition (dominated by furanic diterpenes, alkaloids, sterols, and iridoids) was proved to present a complex biological activity, with cardioprotective, antioxidant, antimicrobial, anti-inflammatory, analgesic, nephroprotective, and antiviral properties, among others.
The current study aimed to present the progress made in the study of motherwort from the date of the European Medicine Agency “Assessment report on Leonurus cardiaca L., herba”. According to our findings, most of the literature data focuses on cardioprotective and antioxidant potential of L. cardiaca, although the data also suggest the exploration of new applications. This, in turn, would be a promising research area for future studies. Future research should also be focused on a definitive conclusion regarding the composition of motherwort (especially the presence of leonurine), as well as the opening of new research directions, such as the use of L. cardiaca extracts in nanotechnology (for the phytosynthesis of nanoparticles).
Acknowledgments
The authors gratefully acknowledge the support obtained through the project SusMAPWaste, SMIS 104323, Contract No. 89/09.09.2016, from the Operational Program Competitiveness 2014-2020, project cofinanced from the European Regional Development Fund.
Conflicts of Interest
The authors declare no conflicts of interest.
Authors' Contributions
All authors contributed equally to data collection and analysis, and manuscript design. Irina Fierascu and Radu Claudiu Fierascu prepared and revised the manuscript.
References
- 1.Bojor O. Romanian: Ghidul Plantelor Medicinale şi Aromatice de la A la Z. Bucharest, Romania: Fiat Lux; 2003. Guide of medicinal and aromatic plants from A to Z; pp. 94–95. [Google Scholar]
- 2.Dong Y., Liao J., Yao K., Jiang W., Wang J. Application of Traditional Chinese medicine in treatment of atrial fibrillation. Evidence-Based Complementary and Alternative Medicine. 2017;2017:11. doi: 10.1155/2017/1381732.1381732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Menkovic N., Šavikin K., Zdunic G., et al. Medicinal plants in northern Montenegro: traditional knowledge, quality, and resources. In: Pieroni A., Quave C. L., editors. Ethnobotany and Biocultural Diversities in the Balkans: Perspectives on Sustainable Rural Development and Reconciliation. New York, NY, USA: Springer-Verlag; 2014. pp. 197–228. [Google Scholar]
- 4.Shikov A. N., Pozharitskaya O. N., Makarov V. G., Wagner H., Verpoorte R., Heinrich M. Medicinal plants of the Russian pharmacopoeia; their history and applications. Journal of Ethnopharmacology. 2014;154(3):481–536. doi: 10.1016/j.jep.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 5.European Medicines Agency. Assessment report on Leonurus cardiaca L., herba. https://www.ema.europa.eu/documents/herbal-report/final-assessment-report-leonurus-cardiaca-l-herba_en.pdf, 2018.
- 6.Shikov A. N., Tsitsilin A. N., Pozharitskaya O. N., Makarov V. G., Heinrich M. Traditional and current food use of wild plants listed in the Russian pharmacopoeia. Frontiers in Pharmacology. 2017;8:p. 841. doi: 10.3389/fphar.2017.00841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rusch C., Hennig L., Rauwald H. 7-Chloro-6-desoxy-harpagide, a major iridoid glucoside from Leonurus cardiaca L. (Ph. Eur.) Planta Medica. 2010;76(12) doi: 10.1055/s-0030-1264533.P235 [DOI] [Google Scholar]
- 8.Kuchta K., Ortwein J., Savtschenko A., Briel D., Volk R., Rauwald H. Leonurus cardiaca, L. Japonicus, Leonotis leonurus: Quantitative HPLC and instrumental HPTLC determination of fourteen phenolics. Planta Medica. 2012;78(11) doi: 10.1055/s-0032-1321210.1259 [DOI] [Google Scholar]
- 9.Kuchta K., Volk R. B., Rauwald H. W. Stachydrine in Leonurus cardiaca, Leonurus japonicus, Leonotis leonurus: detection and quantification by instrumental HPTLC and 1H-qNMR analyses. Die Pharmazie. 2013;68(7):534–540. doi: 10.1691/ph.2013.6527. [DOI] [PubMed] [Google Scholar]
- 10.Kuchta K., Ortwein J., Hennig L., Rauwald H. W. 1H-qNMR for direct quantification of stachydrine in Leonurus japonicus and L. cardiaca. Fitoterapia. 2014;96:8–17. doi: 10.1016/j.fitote.2014.03.023. [DOI] [PubMed] [Google Scholar]
- 11.Azimova S. S., Glushenkova A. I. Leonurus cardiaca L. In: Azimova S. S., Glushenkova A. I., Vinogradova V. I., editors. Lipids, Lipophilic Components and Essential Oils from Plant Sources. London, UK: Springer-Verlag; 2012. pp. 425–426. [DOI] [Google Scholar]
- 12.Zhogova A. A., Perova I. B., Samylina I. A., Eller K. I., Ramenskaya G. V. Identification and quantitative determination of the main biologically active substances in motherwort herb by HPLC–mass spectrometry. Pharmaceutical Chemistry Journal. 2014;48(7):461–466. doi: 10.1007/s11094-014-1132-5. [DOI] [Google Scholar]
- 13.Tahmouzi S., Ghodsi M. Optimum extraction of polysaccharides from motherwort leaf and its antioxidant and antimicrobial activities. Carbohydrate Polymers. 2014;112:396–403. doi: 10.1016/j.carbpol.2014.06.024. [DOI] [PubMed] [Google Scholar]
- 14.Qi M., Yin L., Xu L., et al. Dioscin alleviates lipopolysaccharide-induced inflammatory kidney injury via the microRNA let-7i/TLR4/MyD88 signaling pathway. Pharmacological Research. 2016;111:509–522. doi: 10.1016/j.phrs.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 15.Rauwald H. W., Savtschenko A., Merten A., Rusch C., Appel K., Kuchta K. GABAA receptor binding assays of standardized leonurus cardiaca and leonurus japonicus extracts as well as their isolated constituents. Planta Medica. 2015;81(12-13):1103–1110. doi: 10.1055/s-0035-1546234. [DOI] [PubMed] [Google Scholar]
- 16.Ritter M., Melichar K., Strahler S., et al. Cardiac and electrophysiological effects of primary and refined extracts from Leonurus cardiaca L. (Ph.Eur.) Planta Medica. 2010;76(6):572–582. doi: 10.1055/s-0029-1240602. [DOI] [PubMed] [Google Scholar]
- 17.Liobikas J., Majiene D., Trumbeckaite S., et al. Uncoupling and antioxidant effects of ursolic acid in isolated rat heart mitochondria. Journal of Natural Products. 2011;74(7):1640–1644. doi: 10.1021/np200060p. [DOI] [PubMed] [Google Scholar]
- 18.Bernatoniene J., Kopustinskiene D. M., Jakstas V., et al. The effect of Leonurus cardiaca herb extract and some of its flavonoids on mitochondrial oxidative phosphorylation in the heart. Planta Medica. 2014;80(7):525–532. doi: 10.1055/s-0034-1368426. [DOI] [PubMed] [Google Scholar]
- 19.Shikov A. N., Pozharitskaya O. N., Makarov V. G., Demchenko D. V., Shikh E. V. Effect of Leonurus cardiaca oil extract in patients with arterial hypertension accompanied by anxiety and sleep disorders. Phytotherapy Research. 2011;25(4):540–543. doi: 10.1002/ptr.3292. [DOI] [PubMed] [Google Scholar]
- 20.Xie X., Zhang Z., Wang X., et al. Stachydrine protects eNOS uncoupling and ameliorates endothelial dysfunction induced by homocysteine. Molecular Medicine. 2018;24(1) doi: 10.1186/s10020-018-0010-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goetz P. Apport de la phytothérapie au traitement des troubles du rythme cardiaque. Phytothérapie. 2013;11(3):178–180. doi: 10.1007/s10298-013-0784-8. [DOI] [Google Scholar]
- 22.Goetz P. Synoptique des plantes à effet cardiovasculaire. Phytothérapie. 2013;11(3):181–187. doi: 10.1007/s10298-013-0785-7. [DOI] [Google Scholar]
- 23.Zaurov D. E., Belolipov I. V., Kurmukov A. G., Sodombekov I. S., Akimaliev A. A., Eisenman S. W. The medicinal plants of Uzbekistan and Kyrgyzstan. In: Eisenman S. W., Zaurov D. E., Struwe L., editors. Medicinal Plants of Central Asia: Uzbekistan and Kyrgyzstan. New York, NY, USA: Springer-Verlag; 2013. pp. 15–273. [DOI] [Google Scholar]
- 24.Brenyo A., Aktas M. K. Review of complementary and alternative medical treatment of arrhythmias. American Journal of Cardiology. 2014;113(5):897–903. doi: 10.1016/j.amjcard.2013.11.044. [DOI] [PubMed] [Google Scholar]
- 25.Kidd J. R. Alternative medicines for the geriatric veterinary patient. Veterinary Clinics of North America - Small Animal Practice. 2012;42(4):809–822. doi: 10.1016/j.cvsm.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Jarić S., MačUkanović-Jocić M., Djurdjević L., et al. An ethnobotanical survey of traditionally used plants on Suva planina mountain (south-eastern Serbia) Journal of Ethnopharmacology. 2015;175:93–108. doi: 10.1016/j.jep.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Suroowan S., Mahomoodally F. Common phyto-remedies used against cardiovascular diseases and their potential to induce adverse events in cardiovascular patients. Clinical Phytoscience. 2015;1(1) doi: 10.1186/s40816-015-0002-3. [DOI] [Google Scholar]
- 28.Wang D., Wang J., Liu Y., Zhao Z., Liu Q. Roles of Chinese herbal medicines in ischemic heart diseases (IHD) by regulating oxidative stress. International Journal of Cardiology. 2016;220:314–319. doi: 10.1016/j.ijcard.2016.06.161. [DOI] [PubMed] [Google Scholar]
- 29.Madridejos Mora R. Efectos de las plantas medicinales en los pacientes afectados de insuficiencia cardíaca. FMC - Formación Médica Continuada en Atención Primaria. 2016;23(7):420–429. doi: 10.1016/j.fmc.2016.01.008. [DOI] [Google Scholar]
- 30.Yarnell E. Herbs for atrial fibrillation. Alternative and Complementary Therapies. 2017;23(3):102–111. doi: 10.1089/act.2017.29114.eya. [DOI] [Google Scholar]
- 31.Orhan I. E., Gokbulut A., Senol F. S. Adonis sp., Convallaria sp., Strophanthus sp., thevetia sp., and Leonurus sp. - cardiotonic plants with known traditional use and a few preclinical and clinical studies. Current Pharmaceutical Design. 2017;23(7):1051–1059. doi: 10.2174/1381612822666161010104548. [DOI] [PubMed] [Google Scholar]
- 32.Bianchi I. Herbal medicine in cardiology. In: Fioranelli M., editor. Integrative Cardiology, a New Therapeutic Vision. Springer International Publishing; 2017. pp. 3–18. [DOI] [Google Scholar]
- 33.Song X., Wang T., Zhang Z., et al. Leonurine exerts anti-inflammatory effect by regulating inflammatory signaling pathways and cytokines in LPS-induced mouse mastitis. Inflammation. 2014;38(1):79–88. doi: 10.1007/s10753-014-0009-9. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y., Duan C., Chen H., et al. Inhibition of COX-2/mPGES-1 and 5-LOX in macrophages by leonurine ameliorates monosodium urate crystal-induced inflammation. Toxicology and Applied Pharmacology. 2018;351:1–11. doi: 10.1016/j.taap.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 35.Sadowska B., Micota B., Rozalski M., Redzynia M., Rozalski M. The immunomodulatory potential of Leonurus cardiaca extract in relation to endothelial cells and platelets. Journal of Innate Immunity. 2017;23(3):285–295. doi: 10.1177/1753425917691116. [DOI] [PubMed] [Google Scholar]
- 36.Flemmig J., Noetzel I., Arnhold J., Rauwald H.-W. Leonurus cardiaca L. herb extracts and their constituents promote lactoperoxidase activity. Journal of Functional Foods. 2015;17:328–339. doi: 10.1016/j.jff.2015.05.039. [DOI] [Google Scholar]
- 37.Micota B., Sadowska B., Podsedek A., Redzynia M., Rózalska B. Leonurus cardiaca L. herb - a derived extract and an ursolic acid as the factors affecting the adhesion capacity of Staphylococcus aureus in the context of infective endocarditis. Acta Biochimica Polonica. 2014;61(2):385–388. [PubMed] [Google Scholar]
- 38.Samoilova Z., Muzyka N., Lepekhina E., Oktyabrsky O., Smirnova G. Medicinal plant extracts can variously modify biofilm formation in Escherichia coli. Antonie van Leeuwenhoek-Journal of Microbiology. 2014;105(4):709–722. doi: 10.1007/s10482-014-0126-3. [DOI] [PubMed] [Google Scholar]
- 39.Micota B., Sadowska B., Podsȩdek A., Paszkiewicz M., Sosnowska D., Różalska B. Is it true that plant-derived polyphenols are always beneficial for the human? In vitro study on Leonurus cardiaca extract properties in the context of the pathogenesis of Staphylococcus aureus infections. Journal of Medical Microbiology. 2016;65(10):1171–1181. doi: 10.1099/jmm.0.000332. [DOI] [PubMed] [Google Scholar]
- 40.Wu H., Dai A., Chen X., et al. Leonurine ameliorates the inflammatory responses in lipopolysaccharide-induced endometritis. International Immunopharmacology. 2018;61:156–161. doi: 10.1016/j.intimp.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 41.Denham A., Green J., Hawkey S. What's in the bottle? Prescriptions formulated by medical herbalists in a clinical trial of treatment during the menopause. Journal of Herbal Medicine. 2011;1(3-4):95–101. doi: 10.1016/j.hermed.2011.07.002. [DOI] [Google Scholar]
- 42.Liu W., Ma S., Pan W., Tan W. Combination of motherwort injection and oxytocin for the prevention of postpartum hemorrhage after cesarean section. The Journal of Maternal-Fetal and Neonatal Medicine. 2016;29(15):2490–2493. doi: 10.3109/14767058.2015.1090425. [DOI] [PubMed] [Google Scholar]
- 43.Romm A., Winston D. History of herbal medicines for women. In: Romm A., editor. Botanical Medicine for Women’s Health. Churchill Livingstone; 2010. pp. 8–23. [DOI] [Google Scholar]
- 44.Romm A., Clare B., Stansbury J. E., et al. Menstrual wellness and menstrual problems. In: Romm A., editor. Botanical Medicine for Women's Health. Churchill Livingstone; 2010. pp. 97–185. [DOI] [Google Scholar]
- 45.Romm A., Weed S. S., Gardiner P., et al. Menopausal health. In: Romm A., editor. Botanical Medicine for Women’s Health. Churchill Livingstone; 2010. pp. 455–520. [DOI] [Google Scholar]
- 46.Lans C., Taylor-Swanson L., Westfall R. Herbal fertility treatments used in North America from colonial times to 1900, and their potential for improving the success rate of assisted reproductive technology. Reproductive Biomedicine & Society Online. 2018;5:60–81. doi: 10.1016/j.rbms.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ebrahimzadeh M. A., Nabavi S. F., Nabavi S. M., Eslami B., Asgarirad H. In vitro antioxidant and free radical scavenging activity of Leonurus cardiaca subsp. Persicus, Grammosciadium platycarpum and Onosma demawendicum. African Journal of Biotechnology. 2010;9(51):8865–8871. [Google Scholar]
- 48.Ebrahimzadeh M. A., Nabavi S. F., Nabavi S. M., Pourmorad F. Nitric oxide radical scavenging potential of some Elburz medicinal plants. African Journal of Biotechnology. 2010;9(32):5212–5217. [Google Scholar]
- 49.Armatu A., Colceru-Mihul S., Bubueanu C., Draghici E., Pirvu L. Evaluation of antioxidant and free scavenging potential of some Lamiaceae species growing in Romania. Romanian Biotechnological Letters. 2010;15(3):5274–5280. [Google Scholar]
- 50.Jafari S., Salaritabar A., Moradi A., Khanavi M., Samadi M. Antioxidant activity and total phenolic content of extracts and fractions of cultivated Leonurus cardiaca L. Planta Medica. 2010;76:p. 376. doi: 10.1055/s-0030-1264674. [DOI] [Google Scholar]
- 51.Yi W., Wetzstein H. Y. Effects of drying and extraction conditions on the biochemical activity of selected herbs. HortScience. 2011;46(1):70–73. doi: 10.21273/HORTSCI.46.1.70. [DOI] [Google Scholar]
- 52.Wong F. C., Yong A. L., Ting E. P., Khoo S. C., Ong H. C., Chai T. T. Antioxidant, metal chelating, anti-glucosidase activities and phytochemical analysis of selected tropical medicinal plants. Iranian Journal of Pharmaceutical Research. 2014;13(4):1409–1415. [PMC free article] [PubMed] [Google Scholar]
- 53.Ji S., Fattahi A., Raffel N., et al. Antioxidant effect of aqueous extract of four plants with therapeutic potential on gynecological diseases; Semen persicae, Leonurus cardiaca, Hedyotis diffusa, and Curcuma zedoaria. European Journal of Medical Research. 2017;22(1) doi: 10.1186/s40001-017-0293-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ziyatdinova G., Snegureva Y., Budnikov H. Novel approach for the voltammetric evaluation of antioxidant activity using DPPH -modified electrode. Electrochimica Acta. 2017;247:97–106. doi: 10.1016/j.electacta.2017.06.155. [DOI] [Google Scholar]
- 55.Ziyatdinova G., Kozlova E., Morozova E., Budnikov H. Chronocoulometric method for the evaluation of antioxidant capacity of medicinal plant tinctures. Analytical Methods. 2018;10(41):4995–5003. doi: 10.1039/C8AY01907J. [DOI] [Google Scholar]
- 56.Sen S., Chakraborty R. The role of antioxidants in human health. In: Andreescu S., Hepel M., editors. Oxidative Stress: Diagnostics, Prevention, and Therapy. Vol. 1083. ACS Symposium Series; 2011. pp. 1–37. [DOI] [Google Scholar]
- 57.Krishnaiah D., Sarbatly R., Nithyanandam R. A review of the antioxidant potential of medicinal plant species. Food and Bioproducts Processing. 2011;89(3):217–233. doi: 10.1016/j.fbp.2010.04.008. [DOI] [Google Scholar]
- 58.Romm A. The Postpartum. In: Romm A., editor. Botanical Medicine for Women’s Health. Churchill Livingstone; 2010. pp. 416–432. [DOI] [Google Scholar]
- 59.Adams M., Schneider S.-V., Kluge M., Kessler M., Hamburger M. Epilepsy in the renaissance: a survey of remedies from 16th and 17th century German herbals. Journal of Ethnopharmacology. 2012;143(1):1–13. doi: 10.1016/j.jep.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 60.Rauwald H., Kuchta K., Savtschenko A., Brückner A., Rusch C., Appel K. GABAA receptor binding assays of standardized Leonurus cardiaca and L. japonicus extracts as well as their isolated constituents. Planta Medica. 2013;79(13) doi: 10.1055/s-0033-1352395. [DOI] [PubMed] [Google Scholar]
- 61.Xu D., Chen M., Ren X., Ren X., Wu Y. Leonurine ameliorates LPS-induced acute kidney injury via suppressing ROS-mediated NF-κB signaling pathway. Fitoterapia. 2014;97:148–155. doi: 10.1016/j.fitote.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 62.Cheng H., Bo Y., Shen W., et al. Leonurine ameliorates kidney fibrosis via suppressing TGF-β and NF-κB signaling pathway in UUO mice. International Immunopharmacology. 2015;25(2):406–415. doi: 10.1016/j.intimp.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 63.Liu C., Yin H., Gao J., Xu X., Zhang T., Yang Z. Leonurine ameliorates cognitive dysfunction via antagonizing excitotoxic glutamate insults and inhibiting autophagy. Phytomedicine. 2016;23(13):1638–1646. doi: 10.1016/j.phymed.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 64.Rezaee-Asl M., Sabour M., Nikoui V., Ostadhadi S., Bakhtiarian A. The study of analgesic effects of Leonurus cardiaca L. in mice by formalin, tail flick and hot plate tests. International Scholarly Research Notices. 2014;2014:5. doi: 10.1155/2014/687697.687697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Todorov D., Hinkov A., Shishkova K., Shishkov S. Antiviral potential of Bulgarian medicinal plants. Phytochemistry Reviews. 2014;13(2):525–538. doi: 10.1007/s11101-014-9357-1. [DOI] [Google Scholar]
- 66.Onumah B. Alternative and complementary treatment of thyroid disorders. In: Wartofsky L., Van Nostrand D., editors. Thyroid Cancer: A Comprehensive Guide to Clinical Management. Van Nostrand Humana Press; 2016. pp. 759–765. [DOI] [Google Scholar]
- 67.Shokri Z., Khoshbin M., Koohpayeh A., et al. Thyroid diseases: pathophysiology and new hopes in treatment with medicinal plants and natural antioxidants. International Journal of Green Pharmacy. 2018;12:S473–S482. [Google Scholar]
- 68.Park Y. A new paradigm shift for the green synthesis of antibacterial silver nanoparticles utilizing plant extracts. Toxicological Research. 2014;30(3):169–178. doi: 10.5487/TR.2014.30.3.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mallory J. Integrative care of the mother-infant dyad. Primary Care: Clinics in Office Practice. 2010;37(1):149–163. doi: 10.1016/j.pop.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 70.Wiesner J., Knöss W. Herbal medicinal products in pregnancy − which data are available? Reproductive Toxicology. 2017;72:142–152. doi: 10.1016/j.reprotox.2017.06.046. [DOI] [PubMed] [Google Scholar]
- 71.Kaye A. D., Baluch A., Kaye A. M. Mineral, vitamin, and herbal supplements. In: Fleisher L. A., editor. Anesthesia and Uncommon Diseases. Sixth. Elsevier Saunders; 2012. pp. 470–487. [DOI] [Google Scholar]
- 72.Anadón A., Martínez-Larrañaga M. R., Ares I., Martínez M. A. Interactions between nutraceuticals/nutrients and therapeutic drugs. In: Gupta R. C., editor. Nutraceuticals. Efficacy, Safety and Toxicity. 1st. Academic Press; 2016. pp. 855–874. [DOI] [Google Scholar]
- 73.Greener M. The hidden problem of herb-drug interactions. Prescriber. 2016;27(9):22–27. doi: 10.1002/psb.1496. [DOI] [Google Scholar]
- 74.Leite P. M., Martins M. A., Castilho R. O. Review on mechanisms and interactions in concomitant use of herbs and warfarin therapy. Biomedicine & Pharmacotherapy. 2016;83:14–21. doi: 10.1016/j.biopha.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 75.Miłkowska-Leyck K., Filipek B., Strzelecka H. Pharmacological effects of lavandulifolioside from Leonurus cardiaca. Journal of Ethnopharmacology. 2002;80(1):85–90. doi: 10.1016/S0378-8741(02)00016-8. [DOI] [PubMed] [Google Scholar]
- 76.Mitchell J. C., Rook A. Botanical Dermatology. Vancouver, B.C., Canada: Greenglass Ltd; 1979. [Google Scholar]
- 77.Zhu Y. P. Chinese Materia Medica: Chemistry, Pharmacology and Applications. Boca Raton, Fla, USA: CRC Press; 1998. [Google Scholar]
- 78.WebMD. Motherwort. https://www.webmd.com/vitamins/ai/ingredientmono-126/motherwort, 2019.
- 79.European Food Safety Authority. Compendium of botanicals reported to contain naturally occuring substances of possible concern for human health when used in food and food supplements. EFSA Journal. 2012;10(5) doi: 10.2903/j.efsa.2012.2663.2663 [DOI] [Google Scholar]