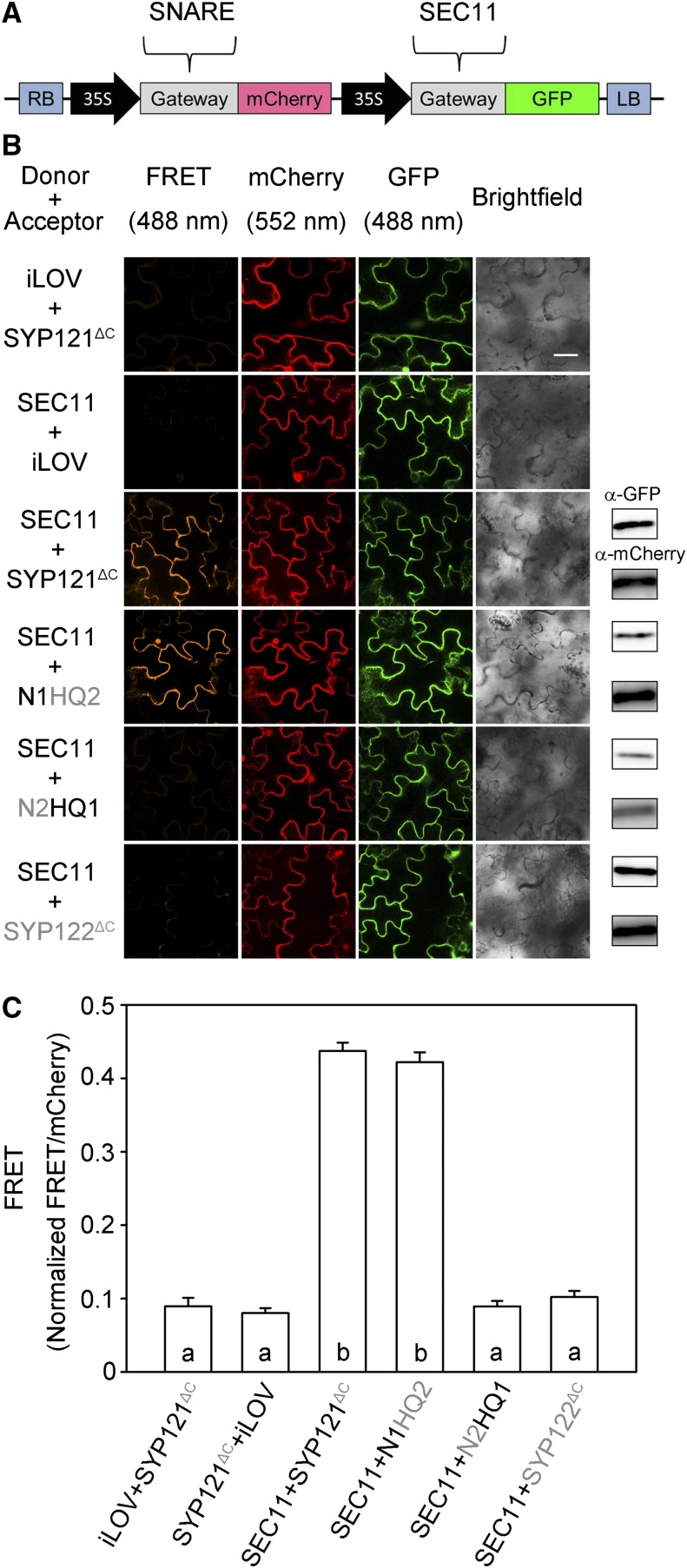
Figure 2.
SEC11 interaction with SYP121 depends on the presence of the N-terminal domain of the SNARE in vivo. FRET analysis was performed for SEC11 interaction with SYP121ΔC, SYP122ΔC, and the SYP121 chimeras. The FRET, mCherry, and GFP fluorescence signals were collected from tobacco (Nicotiana tabacum) leaf epidermis transformed using the 2in1 vector (Hecker et al., 2015). A, Schematic of the 2in1 vectors used for FRET analysis (Hecker et al., 2015). The SEC11-GFP fusion was used as donor, and SNARE-mCherry fusion was used as acceptor. LB, Left border; RB, right border. B, Images at uniform magnification are (left to right) mCherry (FRET) fluorescence (excitation, 488 nm), mCherry fluorescence (excitation, 552 nm), GFP fluorescence (excitation, 488 nm), and bright field. Constructs (top to bottom) expressed iLOV-GFP with SYP121ΔC-mCherry, SEC11-GFP with iLOV-mCherry as negative control, and SEC11-GFP with SYP121ΔC, SYP122 ΔC, and chimeras. Immunoblot analysis is shown at right with anti-GFP (top) and anti-mCherry (below) antibodies to verify fusion protein expression. Bar = 20 μm. C, FRET fluorescence signals from three independent experiments. Each bar represents the mean ± se of fluorescence intensity ratios from 10 images per experiment, taken at random over the root surface. Background fluorescence was subtracted as determined from nontransformed roots. FRET signals were calculated as the mean fluorescence intensity ratio between FRET and mCherry after correcting for bleedthrough and normalizing to the GFP signal. Significant differences (P < 0.05) are indicated by different letters.