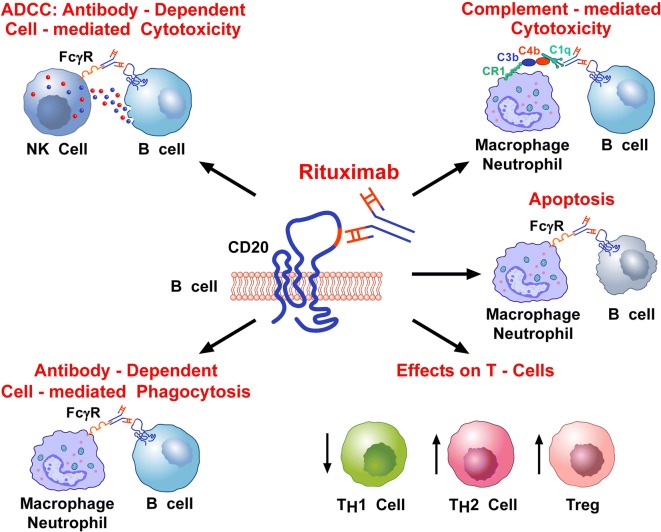
Fig. 1.
Schematic representation of the multiple mechanisms that have been proposed to explain the immunosuppressive effects of RTX. RTX is a chimeric IgG1 monoclonal antibody (mAb) (the Fc domain is humanized, whereas the Fab domain is murine) that targets the CD20 protein on human B-cells. RTX depletes B-cells primarily through an antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-mediated cytotoxicity (CMC). ADCC occurs through the engagement of Fcγ receptor on NK cells by the Fc domain of RTX. Activated NK cells release perforin and granzymes that cause B-cell lysis. CMC occurs through the activation of the complement cascade by the interaction of RTX and CD20, the formation of anaphylatoxins (i.e., C5a, C3a) and the membrane attack complex. The Fcγ receptor is also expressed by human macrophages and neutrophils. Therefore, RTX can also cause antibody-dependent phagocytosis of B-cells by macrophages and neutrophils. Moreover, RTX can cause the apoptosis of B cells through the induction of both CD20 cross-linking on surface membrane and MAP kinase activation. Indeed, apoptotic B-cells are cleared by phagocytes, such as macrophages and neutrophils, through Fcγ receptor’s activation. Finally, there is some evidence that RTX can decrease Th1 cells and can increase Th2 and Treg cells