Abstract
Breast adenoid cystic carcinomas (AdCCs) can pose diagnostic difficulty due to their rarity, particularly on limited biopsy material. Given that these tumors are triple-negative breast cancers with favorable prognosis, accurate diagnosis is critical for clinical management. A total of 12 cases of breast AdCCs were studied; 17 age-matched salivary gland AdCCs and 5 metastatic AdCCs (1 breast and 4 salivary gland primaries) were also examined. Immunohistochemical stains for SOX10, Ki-67, c-KIT, β-catenin, epithelial membrane antigen (EMA), p63, cytokeratin 7 (CK7), cytokeratin 5/6 (CK5/6), and androgen receptor (AR) were performed. All breast (100%) and metastatic (100%) AdCCs and all but 2 salivary gland AdCCs showed diffuse nuclear staining (>50% of cells) for SOX10. Epithelial membrane antigen showed lowest expression in breast AdCCs and the highest expression in metastatic AdCCs (P < .01). Except one case of salivary gland AdCC that showed loss of β-catenin expression and developed subsequent metastasis, all AdCCs showed strong and diffuse membranous β-catenin expression. There were no significant differences in expression of CK7, p63, CK5/6, AR, Ki-67, and c-KIT (P > .05) among breast, salivary gland, and metastatic AdCCs. We investigated the immunophenotypic features of breast AdCCs in comparison with salivary gland and metastatic AdCCs. Despite the contrast in prognosis, these tumors are immunophenotypically similar. SOX10 is a sensitive diagnostic marker in all AdCCs, which could potentially aid in diagnosis of these tumors on limited material.
Keywords: SOX10, breast, adenoid cystic carcinoma
Introduction
Adenoid cystic carcinoma (AdCC) is a malignant tumor that most commonly occurs in the salivary glands,1 but can occasionally present in other organs, including the lung, prostate, skin, and breast.2–5 Unlike its head and neck counterpart, which shows poor long-term outcome,6 breast AdCCs have favorable prognosis.
Histologically, AdCC is characterized by the presence of mixed populations of luminal/epithelial and basal/myoepithelial tumor cells, growing in tubular, cribriform, or solid growth patterns.7,8 Recurrent t(6;9)(q22-23;p23-24) translocation and the formation of the MYB-NFIB fusion gene, resulting in the activation and overexpression of MYB at the mRNA and protein levels is the molecular hallmark of this tumor.1,9,10 A minority of tumors that lack the MYB-NFIB fusion gene likely demonstrate activation of MYB due to molecular mechanisms that are yet unknown.
AdCCs are rare and account for less than 0.1% of all primary carcinomas of the breast.7,11,12 At times, they pose diagnostic difficulty due to their rarity, particularly on limited biopsy samples. Given that AdCC represents a triple-negative breast cancer with favorable prognosis,13 accurate diagnosis of these tumors is critical for appropriate clinical management. Due to the low prevalence of breast AdCC,14,15 its defining features are not well established except that these tumors display a basal-like phenotype13 and are commonly triple negative (estrogen receptor [ER], progesterone receptor [PR], and HER-2 negative).16 On immunohistochemical level, the basal/myoepithelial component expresses basal markers, such as cytokeratin 14 (CK14), cytokeratin 17 (CK17), vimentin, epidermal growth factor receptor (EGFR), and p63, and the luminal/epithelial component is positive for luminal markers, such as cytokeratin 7 (CK7), cytokeratin 8/18 (CK8/18), epithelial membrane antigen (EMA), and c-KIT (CD117).17–20
The SRY-related HMG-box 10 (SOX10) protein is a transcription factor known to be crucial in the specification of the neural crest and maintenance of Schwann cells and melanocytes.21 Expression of SOX10 as a diagnostic marker has been previously established in salivary gland AdCC and basal-like breast carcinoma21,22; however, SOX-10 has not been studied in primary breast AdCC. In this study, we investigated the clinical, histological, and immunophenotypic features of breast AdCC and compared these features with their salivary gland and metastatic counterparts. The goal of our study was to establish SOX-10 as a diagnostic marker in breast AdCCs and to investigate any immunophenotypic differences between breast, salivary gland, and metastatic AdCCs. Insight into the immunophenotype would also help us in understanding their cell of origin and potentially explain the difference in prognosis.
Materials and Methods
Case selection
Approval to perform this study was obtained from the Institutional Review Boards of the Human Studies Protection Office at Washington University (IRB No: 201502060, August 3, 2015). For this retrospective study, the surgical pathology archives of the Washington University School of Medicine (St Louis, MO, USA) and the St. Louis Breast Tumor Registry (MO, USA) were searched for breast primary AdCCs between 1992 and 2014. A total of 12 consecutive cases of breast AdCCs for which formalin-fixed paraffin-embedded (FFPE) blocks were available were obtained and served as the study group; 17 age-matched salivary gland AdCCs and 5 metastatic AdCCs (1 from breast and 4 from salivary gland) were also retrieved and served as the control group. The cases were centrally reviewed and the diagnoses were confirmed in consensus by 3 pathologists (CY, LZ, and SS) using current diagnostic criteria.21 Clinical data were collected.
Tissue microarrays
Tissue microarrays (TMAs) of 3.0 mm cores of each case were made in triplicate. Benign tonsil, prostate, and cerebrum tissues were used as controls and for slide orientation.
Immunohistochemistry
Commercially available monoclonal antibodies for SOX10, Ki-67, c-KIT, β-catenin, epithelial membrane antigen (EMA), p63, CK7, cytokeratin 5/6 (CK5/6), and androgen receptor (AR) were used. The immunostain information is summarized in Table 1. Immunohistochemical stains were performed on a Ventana BenchMark XT automatic stainer (Ventana Medical Systems, Inc, Tucson, AZ, USA), following the vendor’s protocol. Adequate positive and negative controls were included for each run.
Table 1.
Clone of antibodies used.
| Antibody name | Clone | Company |
|---|---|---|
| SOX10 | Polyclonal | Cell Marque, Rocklin, CA |
| Ki-67 | 30-9 | Ventana, Tucson, AZ |
| c-KIT | 9.7 | Ventana, Tucson, AZ |
| β-catenin | 14 | Cell Marque, Rocklin, CA |
| EMA | E29 | Ventana, Tucson, AZ |
| p63 | 4A4 | Ventana, Tucson, AZ |
| CK7 | SP52 | Ventana, Tucson, AZ |
| CK5/6 | D5/16B4 | Ventana, Tucson, AZ |
| AR | SP107 | Cell Marque, Rocklin, CA |
Abbreviations: AR, androgen receptor; CK5/6, cytokeratin 5/6; CK7, cytokeratin 7; EMA, epithelial membrane antigen.
The immunohistochemistry (IHC) results were interpreted by 3 pathologists with the following criteria: nuclear staining for SOX10, Ki-67, p63, and AR; membranous staining for EMA and β-catenin; and cytoplasmic staining for CK7, CK5/6, and c-KIT were considered positive. The percentage of cells that were positive was evaluated. For Ki-67, any nuclear reactivity, regardless of intensity, was considered positive. For AR, the Allred scoring system was used (proportion score 0-5 + intensity score 0-3 = total score 0-8; ⩾3 positive result). The percentage of tumor cells labeled was collected and subsequently semi-quantitatively scored as 0 (no tumor cells stained), 1+ (less than 30% of tumor cells stained), 2+ (31%-60% tumor cells stained), 3+ (61%-90% tumor cells stained), and 4+ (greater than 90% tumor cells stained).
Statistical analysis
Welch t test was used to compare the intensity and percentage of tumor cells stained with each marker. A P value of less than .05 was considered statistically significant.
Results
Clinical information is summarized in Tables 2 and 3. For breast AdCC patients, the mean age was 58 years. In all, 11 patients were women and 1 was man. One case of breast AdCC had a positive margin on resection, and all other cases had negative margins. No cases showed recurrence of disease on mean follow-up period of 7.7 years (range: 1-15 years). Histologically, 3 cases showed grade I architecture, 3 cases grade II, and 6 cases grade III with predominant solid architecture. There was no microglandular adenosis associated with any cases. Of cases with known biomarker status, 6 of 9 negative for ER, 8 of 8 negative for PR, and 6 of 6 negative for HER2. Cases with ER positivity exhibited only weak and focal staining with overall Allred score of 3 to 4 of 8.
Table 2.
Clinicopathologic information of breast adenoid cystic carcinoma.
| Case no. | Age (years) | Sex | Specimen type | Side | Size (cm) | Metastasis | Follow-up |
|---|---|---|---|---|---|---|---|
| 1. | 46 | F | Resection | Left | 6.0 | No | 15 years, ANED |
| 2. | 53 | F | Resection | Right | 2.8 | No | 14 years, ANED |
| 3. | 52 | F | Resection | Right | N/A | No | 10 years, ANED |
| 4. | 53 | F | Resection | Right | 0.7 | No | 9 years, ANED |
| 5. | 60 | F | Resection | Left | 1.6 | Lymph node | 1 year, ANED |
| 6. | 54 | F | Resection | N/A | 1.5 | No | 3 years, ANED |
| 7. | 62 | F | Resection | Left | 0.9 | No | 7 years, ANED |
| 8. | 70 | F | Resection | Left | 0.7 | No | 3 years, ANED |
| 9. | 62 | F | Resection | Right | 1.8 | No | 3 years, ANED |
| 10. | 76 | M | Resection | Left | 3.5 | No | 12 years, ANED |
| 11. | N/A | F | Resection | N/A | N/A | No | N/A |
| 12. | N/A | F | Resection | N/A | N/A | No | N/A |
Abbreviation: ANED, alive with no evidence of disease; F, female; M, male.
Table 3.
Clinical comparison of adenoid cystic carcinoma of breast, salivary gland, and metastatic AdCC.
| Breast (N = 12) | Salivary (N = 17) | Metastatic (N = 5) | |
|---|---|---|---|
| Mean age, years (range) | 58 (46-76) | 53 (36-77) | 56 (39-67) |
| Sex | 1 Male/11 females | 5 Males/12 females | 2 Males/3 females |
| Mean size, cm (range) | 2.2 (0.7-6.0) | 4.0 (1.3-6.1) | 3.1 (1.9-3.8) |
| Margin status | 1 (+)/11 (–) | 12 (+)/5 (–) | 0 (+)/1 (–)a |
| Disease-free survival, years (range) | No recurrence | 2.5 (0-7) | 2.5 (0-5) |
| Overall survival | No recurrence | NDRTD | 1 death at 28 years |
Abbreviation: NDRTD, no death related to disease.
Four cases were biopsy and margin status is not applicable.
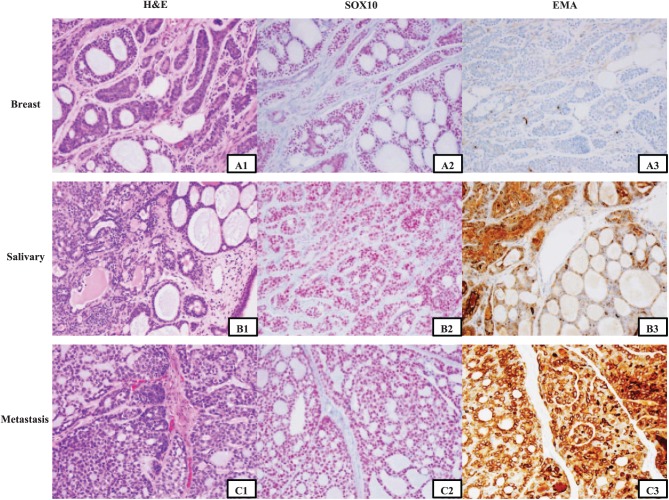
IHC results are summarized in Tables 4 and 5. All cases of breast (100%) and metastatic (100%) AdCCs and all but 2 cases of salivary AdCCs showed diffuse nuclear staining (>50% of cells) for SOX10 (Figure 1). SOX10 is nondiscriminatorily expressed in both the epithelial and myoepithelial cells. And the staining pattern did not differ with the architecture grade of the tumor. Epithelial membrane antigen expression was different in breast, salivary gland, and metastatic AdCCs; the lowest expression (both percentage and intensity) was seen in breast AdCCs and the highest expression in metastatic AdCCs (P < .01). Except one case of salivary primary AdCC, which showed loss of β-catenin expression and developed subsequent metastasis to the T10 vertebra, all cases of AdCCs showed strong and diffuse membrane β-catenin expression. Androgen receptor (AR) staining in AdCCs showed a heterogeneic pattern, more intensive in basal cells than the luminal cells. The Ki-67 labeling index of breast AdCC ranged from 1% to 20%, with an average of 7%, not statistically increased compared with salivary gland AdCCs. There was also slight increase in Ki-67 index for metastatic AdCCs (P > .05). There were no significant differences in expression of CK7, p63, CK5/6, and c-KIT (P > .05) among breast, salivary gland, and metastatic AdCCs.
Table 4.
Immunohistochemistry results for breast adenoid cystic carcinoma.
| Case | SOX10 (%) | c-KIT (%) | β-catenin (%) | EMA (%) | p63 (%) | CK7 (%) | CK5/6 (%) | ARa (%) |
|---|---|---|---|---|---|---|---|---|
| 1. | 4+ (95) | 3+ (63) | 4+ (100) | 0 (0) | 3+ (87) | 3+ (88) | 4+ (100) | 3 (5) |
| 2. | 4+ (100) | 3+ (87) | 4+ (100) | 0 (0) | 1+ (2) | 2+ (50) | 1+ (14) | 4 (20) |
| 3. | 4+ (100) | 1+ (30) | 4+ (100) | 1+ (2) | 1+ (3) | 4+ (100) | 0 (0) | 4 (30) |
| 4. | 4+ (100) | 4+ (100) | 4+ (100) | 0 (0) | 1+ (6) | 2+ (53) | 1+ (4) | 2 (1) |
| 5. | 4+ (100) | 2+ (40) | 4+ (100) | 1+ (1) | 1+ (2) | 4+ (100) | 2+ (43) | 0 (0) |
| 6. | 3+ (78) | 2+ (45) | 4+ (100) | 1+ (0) | 4+ (93) | 3+ (80) | 3+ (73) | 4 (30) |
| 7. | 4+ (93) | 1+ (28) | 4+ (100) | 1+ (0) | 3+ (83) | 1+ (15) | 4+ (100) | 4 (20) |
| 8. | 4+ (100) | 3+ (90) | 4+ (100) | 1+ (4) | 3+ (73) | 4+ (100) | 4+ (100) | 3 (10) |
| 9. | 3+ (65) | 1+ (25) | 4+ (100) | 1+ (5) | 3+ (88) | 2+ (57) | 3+ (90) | 4 (20) |
| 10. | 4+ (100) | 4+ (100) | 4+ (100) | 1+ (17) | 2+ (37) | 4+ (100) | 4+ (100) | 5 (30) |
| 11. | 4+ (100) | 4+ (93) | 4+ (100) | 1+ (2) | 3+ (67) | 4+ (100) | 1+ (22) | 4 (20) |
| 12. | 4+ (100) | 3+ (70) | 4+ (100) | 1+ (4) | 3+ (73) | 3+ (75) | 3+ (80) | 0 (0) |
Abbreviations: AR, androgen receptor.
indicates no tumor cells stained; 1+, ⩽30% tumor cells stained; 2+, 31%-60%; 3+, 61%-90%; 4+, >90%.
The AR expression is interpreted using the Allred scoring system.
Table 5.
Comparison of staining pattern in AdCC.
| Breast AdCC |
Salivary AdCC |
Metastatic AdCC |
||||
|---|---|---|---|---|---|---|
| Percentage (%) | Mean intensity (range) | Percentage (%) | Mean intensity (range) | Percentage (%) | Mean intensity (range) | |
| SOX10 | 93 (63-100) | 2.23 (1-3) | 83 (19-100) | 2.14 (1-3) | 96 (75-100) | 2.50 (2-3) |
| c-KIT | 68 (25-100) | 2.19 (1-3) | 61 (13-100) | 1.94 (1-3) | 50 (15-87) | 1.67 (1-2) |
| β-catenin | 100 (100) | 2.72 (2-3) | 94 (30-100) | 2.41 (1-3) | 100 (100) | 2.50 (2-3) |
| EMA | 3 (0-17) | 0.72 (1-3) | 12 (1-52) | 1.49 (1-3) | 34 (1-93) | 2.00 (1-3) |
| P63 | 56 (2-88) | 2.55 (2-3) | 64 (1-97) | 2.43 (1-3) | 50 (2-77) | 1.83 (1-3) |
| CK7 | 73 (15-100) | 2.45 (1-3) | 82 (6-100) | 2.67 (2-3) | 81 (25-100) | 3.00 (3) |
| CK5/6 | 66 (0-100) | 2.29 (1-3) | 79 (50-100) | 2.43 (1-3) | 50 (5-65) | 2.25 (1-3) |
| Ki-67 | 7 (1-20) | N/A | 6 (1-27) | N/A | 12 (5-25) | N/A |
| ARa | 1.9 (0-5) | 1.17 (0-3) | 2.02 (0-5) | 0.84 (0-2) | 1.58 (0-5) | 0.58 (0-2) |
Abbreviation: AdCC, adenoid cystic carcinoma.
AR is interpreted using the Allred scoring system (proportion score: 0-5; intensity score 0-3).
Figure 1.
Immunohistochemistry staining for SOX10 and EMA in adenoid cystic carcinoma. The characteristic cribriform pattern for adenoid cystic carcinoma is shown in A1, B1, and C1. Strong and diffuse staining of SOX10 (red chromogen) for adenoid cystic carcinoma in both epithelial and myoepithelial cells is observed in breast, salivary gland, and metastatic cases (A2, B2, and C2). EMA (brown chromogen) is the only marker with gradient staining among breast, salivary gland, and metastatic adenoid cystic carcinomas. Only single cells are stained in this case of breast adenoid cystic carcinoma (A3), whereas there is more diffuse staining seen in the salivary gland tumor (B3) and diffuse staining in the metastatic tumor (C3). EMA indicates epithelial membrane antigen.
Discussion
Our study reinforces the indolent nature of AdCCs of the breast, as all 12 cases of breast AdCC in our study showed no evidence of metastasis to distant sites on routine clinical follow-up. Only one patient had a regional lymph node metastasis at the time of diagnosis, but was free of disease on 1-year follow-up. Even if we consider this case as one with metastatic disease, the prognosis for breast AdCC is still better than those reported for salivary gland AdCC. Our cohort also included a unique case of male patient with breast AdCC. This case showed identical clinical and histological characteristics as other cases of breast AdCCs. The patient did not show any evidence of recurrence during 12 years of follow-up. In explaining the different behavior of salivary AdCC and breast AdCC, one thing to consider is that most cases of breast AdCCs are resected with clear margin status given the anatomic approachability. However, the surrounding structures in the head and neck (eg, vessels, nerves, bones) make resection with clear margins challenging, if not impossible. As a result, the margins in salivary AdCCs are commonly positive and could potentially be one reason for residual disease.
The SRY-related HMG-box 10 (SOX10) protein is a transcription factor known to be crucial in the specification of the neural crest and maintenance of Schwann cells and melanocytes.21 Expression of SOX10 as a diagnostic marker has been previously established in salivary gland AdCC, basal-like breast carcinoma, and benign breast myoepithelial cells21,22; however, SOX-10 has not been studied in primary breast AdCC, especially in comparison with salivary AdCC. We demonstrated that SOX10 is a sensitive diagnostic marker in all AdCCs being expressed diffusely (>50% of cells) in both the luminal and basal layers in 100% of cases of breast and metastatic AdCCs. Specifically, in cases of breast AdCC, most of the cases showed greater than 90% of tumor cell positivity for SOX10 (10 of 12 cases), where the remaining 2 cases showed 60% to 90% of tumor cells positive for SOX10. This is extremely helpful when interpreting needle core biopsies or fine-needle aspiration, where only limited tissue is sampled. The fact that SOX10 stained both the luminal and basal layer cell population is also helpful in that some cases of AdCCs show a dominant component of one single cell lineage. In addition, the nuclear staining pattern makes interpretation of staining much easier on limited biopsy material and fine-needle aspiration samples. While SOX10 is sensitive in the breast AdCCs, its utility might be limited when the differential diagnosis includes basal-like breast carcinomas, metastatic melanoma, and a small subset of breast invasive ductal carcinomas.13,23
In our study, we found a difference in both percentage of stained cells and intensity of staining for EMA in AdCCs of the breast when compared with other AdCCs. Noticeably, EMA showed intensive staining in metastatic AdCCs and weakest staining in breast AdCCs. Whether this pattern is prognostically significant is debatable, as the percentage of cells staining in primary lesions is low and hard to interpret on a daily sign-out basis. However, it is possible that with metastatic disease, the EMA expression increases in tumor cells. It would be helpful to assess this difference in expression in paired primary-metastatic samples from the same patient. Our case series lacked such paired tumor-normal samples, and this finding could not be investigated further in our study.
β-catenin showed cytoplasmic and membranous staining in all but one case of AdCC. The only case that lost expression of β-catenin was from a patient with salivary gland AdCC that later developed metastasis to the T10 vertebra. This finding is consistent with other studies that have shown loss of membranous β-catenin expression to be a poor prognostic factor in salivary gland AdCCs.24–26 Of note, the β-catenin status for the metastatic lesion in our case is unknown.
Other stains including c-KIT, p63, CK7, CK5/6, and AR all showed similar staining patterns among the 3 groups, suggesting that these tumors share more in common than just histologic features. There was a slight increase in Ki-67 proliferation index for the metastatic AdCCs. Previous studies have concluded Ki-67 proliferation index above 4% as a poor prognostic factor for AdCCs of the salivary gland.27 Androgen receptor has been shown to be variously expressed in normal breast ductal epithelium, serving as a good internal positive control. It is also usually expressed in a high proportion of breast ductal carcinomas; therefore, it cannot be used to confirm the diagnosis of AdCC versus invasive ductal carcinoma in this setting. In the head and neck regions, salivary duct carcinomas also show AR reactivity,28 and AR is used as a diagnostic tool in that setting. Thus, in the salivary glands when the differential diagnosis includes AdCCs, AR should be interpreted with caution and may be a potential diagnostic pitfall. A recent study showed that SOX10 had negative correlation with AR in triple-negative breast carcinomas. However, such correlation was not shown in our study.29
In summary, we investigated the immunophenotypic features of breast AdCCs in comparison with salivary gland and metastatic AdCCs. Despite the contrast in prognosis, our results indicate that these lesions have more in common immunophenotypically. SOX10 is sensitive in all AdCCs, which could potentially be helpful in diagnosis.
Footnotes
Declaration of Conflicting Interests:The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: This manuscript is an original article and has not been submitted for publication elsewhere. Part of this work was poster presented at the United States and Canadian Academy of Pathology Meeting, March 12-18, 2016, Seattle, Washington, USA. Dr Souzan Sanati is a consultant for Genentech. None of the authors have any relationships with, or financial interest in, any commercial companies pertaining to this article.
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the discretionary funds from the Department of Pathology and Immunology, Washington University School of Medicine, St Louis, MO, USA.
Author Contributions: CY (MD) has contributed to the study design, acquisition and interpretation of data, data analysis, and drafting the manuscript. LZ (MD) has contributed to data acquisition. SS (MD) has contributed to interpretation of data and review and modification of the manuscript.
ORCID iD: Chen Yang  https://orcid.org/0000-0002-6371-5930
https://orcid.org/0000-0002-6371-5930
References
- 1. Marchio C, Weigelt B, Reis-Filho JS. Adenoid cystic carcinomas of the breast and salivary glands (or “The strange case of Dr. Jekyll and Mr. Hyde” of exocrine gland carcinomas). J Clin Pathol. 2010;63:220–228. [DOI] [PubMed] [Google Scholar]
- 2. Maziak DE, Todd TR, Keshavjee SH, Winton TL, Van Nostrand P, Pearson FG. Adenoid cystic carcinoma of the airway: thirty-two-year experience. J Thorac Cardiovasc Surg. 1996;112:1522–1531; discussion 1531. [DOI] [PubMed] [Google Scholar]
- 3. Grignon DJ, Ro JY, Ordonez NG, Ayala AG, Cleary KR. Basal cell hyperplasia, adenoid basal cell tumor, and adenoid cystic carcinoma of the prostate gland: an immunohistochemical study. Hum Pathol. 1988;19:1425–1433. [DOI] [PubMed] [Google Scholar]
- 4. Wick MR, Swanson PE. Primary adenoid cystic carcinoma of the skin: a clinical, histological, and immunocytochemical comparison with adenoid cystic carcinoma of salivary glands and adenoid basal cell carcinoma. Am J Dermatopathol. 1986;8:2–13. [DOI] [PubMed] [Google Scholar]
- 5. Ro JY, Silva EG, Gallager HS. Adenoid cystic carcinoma of the breast. Human Pathol. 1987;18:1276–1281. [DOI] [PubMed] [Google Scholar]
- 6. Nascimento AG, Amaral AL, Prado LA, et al. Adenoid cystic carcinoma of salivary glands. A study of 61 cases with clinicopathologic correlation. Cancer. 1986;57:312–319. [DOI] [PubMed] [Google Scholar]
- 7. Sapino A, Sneige N, Eusebi V. Adenoid cystic carcinoma. In: Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ, eds. World Health Organization Classification of Tumours of the Breast. 4th ed. Lyon: IARC Press; 2012:56–57. [Google Scholar]
- 8. Szanto PA, Luna MA, Tortoledo ME, White RA. Histologic grading of adenoid cystic carcinoma of the salivary glands. Cancer. 1984;54:1062–1069. [DOI] [PubMed] [Google Scholar]
- 9. Persson M, Andren Y, Mark J, Horlings HM, Persson F, Stenman G. Recurrent fusion of MYB and NFIB transcription factor genes in carcinomas of the breast and head and neck. Proc Natl Acad Sci U S A. 2009;106:18740–18744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brill LB, 2nd, Kanner WA, Fehr A, et al. Analysis of MYB expression and MYB-NFIB gene fusions in adenoid cystic carcinoma and other salivary neoplasms. Mod Pathol. 2011;24:1169–1176. [DOI] [PubMed] [Google Scholar]
- 11. Rosen PP. Adenoid cystic carcinoma of the breast: a morphologically heterogeneous neoplasm. Pathol Annu. 1989;24:237–254. [PubMed] [Google Scholar]
- 12. Glazebrook KN, Reynolds C, Smith RL, et al. Adenoid cystic carcinoma of the breast. Am J Roentgenol. 2010;194:1391–1396. [DOI] [PubMed] [Google Scholar]
- 13. Wetterskog D, Lopez-Garcia MA, Lambros MB, et al. Adenoid cystic carcinomas constitute a genomically distinct subgroup of triple-negative and basal-like breast cancers. J Pathol. 2012;226:84–96. [DOI] [PubMed] [Google Scholar]
- 14. Ghabach B, Anderson WF, Curtis RE, Huycke MM, Lavigne JA, Dores GM. Adenoid cystic carcinoma of the breast in the United States (1977 to 2006): a population-based cohort study. Breast Cancer Res. 2010;12:R54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kulkarni N, Pezzi CM, Greif JM, et al. Rare breast cancer: 933 adenoid cystic carcinomas from the National Cancer Data Base. Ann Surg Oncol. 2013;20:2236–2241. [DOI] [PubMed] [Google Scholar]
- 16. Weigelt B, Horlings HM, Kreike B, et al. Refinement of breast cancer classification by molecular characterization of histological special types. J Pathol. 2008;216:141–150. [DOI] [PubMed] [Google Scholar]
- 17. Azoulay S, Lae M, Freneaux P, et al. KIT is highly expressed in adenoid cystic carcinoma of the breast, a basal-like carcinoma associated with a favorable outcome. Mod Pathol. 2005;18:1623–1631. [DOI] [PubMed] [Google Scholar]
- 18. Crisi GM, Marconi SA, Makari-Judson G, et al. Expression of c-kit in adenoid cystic carcinoma of the breast. Am J Clin Pathol. 2005;124:733–739. [DOI] [PubMed] [Google Scholar]
- 19. Mastropasqua MG, Maiorano E, Pruneri G, et al. Immunoreactivity for c-kit and p63 as an adjunct in the diagnosis of adenoid cystic carcinoma of the breast. Mod Pathol. 2005;18:1277–1282. [DOI] [PubMed] [Google Scholar]
- 20. Chen JC, Gnepp DR, Bedrossian CW. Adenoid cystic carcinoma of the salivary glands: an immunohistochemical analysis. Oral Surg Oral Med Oral Pathol. 1988;65:316–326. [DOI] [PubMed] [Google Scholar]
- 21. Ohtomo R, Mori T, Shibata S, et al. SOX10 is a novel marker of acinus and intercalated duct differentiation in salivary gland tumors: a clue to the histogenesis for tumor diagnosis. Mod Pathol. 2013;26:1041–1050. [DOI] [PubMed] [Google Scholar]
- 22. Ivanov SV, Panaccione A, Nonaka D, et al. Diagnostic SOX10 gene signatures in salivary adenoid cystic and breast basal-like carcinomas. Br J Cancer. 2013;109:444–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mohamed A, Gonzalez RS, Lawson D, Wang J, Cohen C. SOX10 expression in malignant melanoma, carcinoma, and normal tissues. Appl Immunohistochem Mol Morphol. 2013;21:506–510. [DOI] [PubMed] [Google Scholar]
- 24. Ferrazzo KL, Neto MM, Dos Santos E, et al. Differential expression of galectin-3, β-catenin, and cyclin D1 in adenoid cystic carcinoma and polymorphous low-grade adenocarcinoma of salivary glands. J Oral Pathol Med. 2009;38:701–707. [DOI] [PubMed] [Google Scholar]
- 25. Schneider S, Thurnher D, Seemann R, et al. The prognostic significance of β-catenin, cyclin D1 and PIN1 in minor salivary gland carcinoma: β-catenin predicts overall survival. Eur Arch Otorhinolaryngol. 2016;273:1283–1292. [DOI] [PubMed] [Google Scholar]
- 26. Dong L, Ge XY, Wang YX, et al. Transforming growth factor-β and epithelial–mesenchymal transition are associated with pulmonary metastasis in adenoid cystic carcinoma. Oral Oncol. 2013;49:1051–1058. [DOI] [PubMed] [Google Scholar]
- 27. Nordgard S, Franzen G, Boysen M, Halvorsen TB. Ki-67 as a prognostic marker in adenoid cystic carcinoma assessed with the monoclonal antibody MIB1 in paraffin sections. Laryngoscope. 1997;107:531–536. [DOI] [PubMed] [Google Scholar]
- 28. Williams L, Thompson LD, Seethala RR, et al. Salivary duct carcinoma: the predominance of apocrine morphology, prevalence of histologic variants, and androgen receptor expression. Am J Surg Pathol. 2015;39:705–713. [DOI] [PubMed] [Google Scholar]
- 29. Harbhajanka A, Chahar S, Miskimen K, et al. Clinicopathological, immunohistochemical and molecular correlation of neural crest transcription factor SOX10 expression in triple-negative breast carcinoma. Hum Pathol. 2018;80:163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]