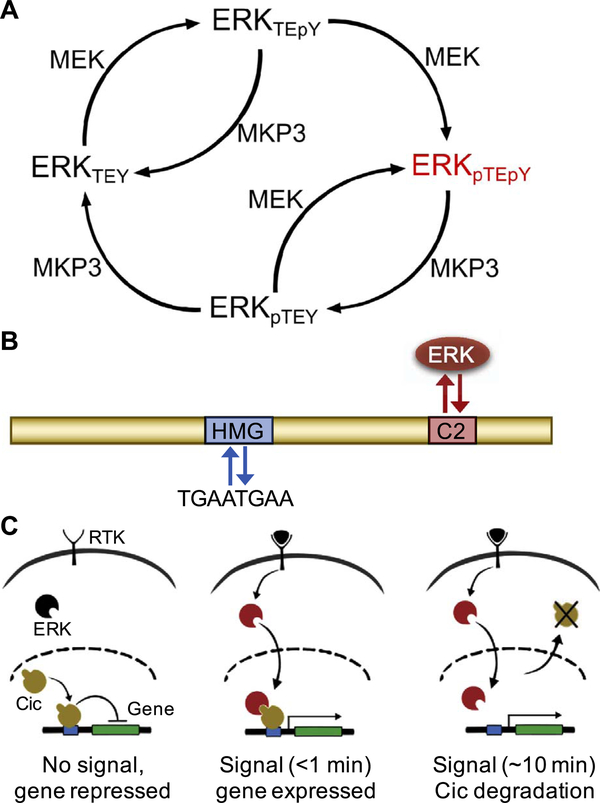
Fig. 2.
Active, dually phosphorylated ERK antagonizes Cic repressor activity. (A) Reaction network depicting the regulation of ERK phosphorylation state by kinase MEK and phosphatase MPK3. Active, dually phosphorylated ERK is depicted in red. (B) Schematic of the Cic protein (yellow) including its major functional domains, high mobility group (HMG) box for DNA binding (blue), and the C2 motif, which functions as a docking site for ERK (red). (C) In response to ligand-induced RTK activation, ERK (black) is phosphorylated; active ERK (red) is translocated to the nucleus, resulting in fast (< 1 min) derepression of the target gene, and cytoplasmic export (~10 min) and subsequent degradation of Cic (yellow).