Abstract
Background:
Sodium retention drives volume overload, with fluid retention largely a passive, secondary phenomenon. However, parameters (urine output, body weight) used to monitor therapy in acute decompensated heart failure (ADHF) measure fluid rather than sodium balance. Thus, the accuracy of fluid-based metrics hinges on the contested assumption that urinary sodium content is consistent.
Objectives:
We sought to better describe sodium excretion in ADHF and to evaluate the prognostic ability of urinary sodium and fluid-based metrics.
Methods:
Patients enrolled in the Renal Optimization Strategies Evaluation-Acute Heart Failure (ROSE-AHF) with 24-hour sodium excretion available were studied (n=316). Patients received protocol-driven high-dose loop diuretic therapy.
Results:
Sodium excretion through the first 24 hours was highly variable (Range: 0.12-19.8g, median: 3.63g, IQR 1.85-6.02g) and was not correlated with diuretic dose (r=0.06, p=0.27). Greater sodium excretion was associated with reduced mortality in a univariate model (HR = 0.80 per doubling of sodium excretion, 95% CI 0.66 – 0.95, p=0.01), whereas gross urine output (p=0.43), net fluid balance (p=0.87), and weight change (p=0.11) were not. Sodium excretion of less than the prescribed dietary sodium intake (2g), even in the setting of a negative net fluid balance, portended a worse prognosis (HR=2.02, 95% CI 1.17–3.46, p=0.01).
Conclusions:
In patients hospitalized with ADHF receiving high-dose loop diuretics, sodium concentration and excretion were highly variable. Sodium excretion was strongly associated with six-month mortality whereas traditional fluid-based metrics were not. Poor sodium excretion, even in the context of fluid loss, portends a worse prognosis.
Keywords: heart failure, sodium excretion, body weight, diuretics
Condensed Abstract:
Sodium retention is the primary process in ADHF, while fluid retention is largely a secondary, passive phenomenon. However, parameters used to assess therapy (weight loss, net fluid balance) measure the latter. The ROSE-AHF trial enrolled a cohort of patients with ADHF receiving high-dose loop diuretics with 24h sodium excretion data (N=316). Urinary sodium concentration was highly variable. Sodium excretion was associated with improved survival, whereas weight and net fluid balance were not. Furthermore, patients with net fluid loss but net sodium gain exhibited worse survival than patients with net sodium loss. Sodium excretion is a promising therapeutic parameter in ADHF.
Introduction
Dysregulation of sodium homeostasis is central to the pathophysiology of heart failure and leads to a positive sodium balance (1-3). Sodium is the primary osmolyte in the extracellular fluid compartment, and the active renal regulation of total body sodium content is utilized by the kidney to passively regulate total body fluid content. Importantly, the downstream effects of sodium retention include fluid retention, which in turn results in the traditional congestive symptoms associated with acute decompensated heart failure (ADHF) (4-7). Loop diuretics, the cornerstone of ADHF therapy, exert their effect by antagonizing renal sodium transporters to increase sodium excretion (8) along with the passive loss of fluid.
Current practice guidelines related to therapeutic monitoring focus on fluid balance and changes in weight (5,6), which serve only as a proxy for sodium excretion. These metrics correlate poorly with each other (7,9), are often difficult to accurately assess, and have not consistently been linked to meaningful outcomes despite their frequent use as endpoints in major clinical trials (10-12). In addition to the fact that fluid and weight loss only indirectly query the physiology of interest (sodium excretion), there is inherent difficulty in obtaining accurate fluid intake and output, even under idealized study conditions (13,14). Furthermore, there is a common assumption that urinary sodium concentration is predictably hypotonic in patients taking a loop diuretic, sometimes compared to the sodium content of “half-normal saline” (15,16). There is an accumulating body of literature challenging the supposition that urinary sodium concentration remains constant throughout the course of a hospitalized patient receiving diuretics (17-19).
Given the central role of sodium in both the pathophysiology and therapy of congestion in ADHF, as well as the known limitations of urine output and weight, we sought to further explore urinary sodium as a potential metric to monitor ADHF treatment. We hypothesized that sodium excretion would be highly variable among patients receiving loop diuretic therapy, making any assumption of a consistent urine sodium concentration, such as “half-normal saline”, unreliable. Next, we hypothesized that higher degrees of sodium excretion, but not weight loss or negative fluid balance, would be associated with improved survival.
Methods
Patient Population
The Renal Optimization Strategies Evaluation in Acute Heart Failure (ROSE-AHF) trial dataset has been previously described (20-22). The data, analytic methods, and study materials are available via the Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC). The original trial consisted of 360 hospitalized ADHF patients with baseline renal dysfunction (estimated glomerular filtration rates (eGFRs) 15-60 mL/min/1.73m2). All patients received open-label, intravenous (IV) loop diuretic treatment. The recommended total daily dose was equal to 2.5 times the total daily oral outpatient furosemide (or equivalent) dose at 7 days prior to admission. The study found no difference in the primary endpoints of cumulative urine output or change in cystatin-C through 72 hours between the intervention groups (low-dose dopamine or low dose nesiritide) and the pooled placebo group (21).
All patients received high-dose loop diuretics as defined above and were placed on a standard 2g/day sodium and 2L/day fluid restriction. Sodium excretion was measured during the three intervention days using 24-hour urine collections, and patients were followed for clinical outcomes through six months after randomization. Our main analysis focuses on the 316 patients with complete data for the first 24 hours (day 1) of sodium excretion. Day 1 sodium excretion was compared to day 2, day 3 (when available), and cumulative 72-hour sodium excretion to assess within patient variability. We focused on data from the first 24 hours (day 1) of the 72-hour intervention period because the first 24 hours after meeting ROSE-AHF inclusion/exclusion criteria was a time when aggressive fluid and sodium removal was a universal therapeutic goal. All patients were by definition volume overloaded at this point since they just met criteria for congestion. We would only expect fluid/sodium balance to track with outcomes if physicians were attempting to achieve a negative sodium or fluid balance. Notably, in the DOSE trial (13) 31% of patients in the high dose arm (same dosing as ROSE-AHF) were switched to oral therapy after 48 hours. In addition, day 1 offered the lowest number of missing data.
Definitions and Calculations
Patients were classified as having a positive sodium balance if their measured sodium excretion on day 1 was less than the amount in the prescribed diet (2g = 87mmol). If they excreted between 2g and 4g (87 – 174mmol) they were considered intermediate responders, and excretion of >4g (174mmol) was deemed to be an excellent response.
The definitions for fluid and weight response were similar to the definitions for sodium. Net fluid balance was calculated from recorded fluid intake and urine output. Patients had a positive fluid balance on day 1 if their fluid intake exceeded their urine output. Intermediate responders for fluid had a negative fluid balance between 0L and 2L negative, and intermediate responders for weight lost between 0kg and 2kg. A net negative fluid balance of 2L or greater was considered excellent, as was a weight loss of greater than 2kg.
To test the assumption that urinary sodium concentration is consistent, such as “half-normal saline”, we defined estimated sodium excretion as a product of total urine output multiplied by the sodium concentration of half-normal saline (77 mmol/L = 1.77 g/L).
Statistical Analyses
The primary outcomes of interest were 1) the variability in 24-hour sodium excretion and 2) six–month all-cause mortality. Categorical variables were compared using the Chi-square test, and continuous variables using student’s T-test, ANOVA, Wilcoxon signed ranks, Mann-Whitney, or Kruskal-Wallis tests based on examination of the distribution. Correlations are reported as Spearman’s rho. The Bland and Altman Method was used to analyze the differences between the measured sodium excretion and estimated sodium excretion using the “half-normal saline” assumption. As the current reference standard, measured sodium excretion was used for the x-axis. However, the bias, or mean of the differences, allowed for an artificially deflated measure of the actual bias at the individual level, so we also calculated the absolute value of the difference between estimated and measured sodium excretion.
Associations between the metrics of diuretic response and six-month all-cause mortality were examined in univariate and multiple regression Cox proportional hazards modeling and through Kaplan-Meier analysis. Multiple regression analyses adjusted for the following baseline characteristics: age, sex, race, heart rate, blood pressure, left ventricular ejection fraction (LVEF), log baseline N-terminal pro-B natriuretic peptide (NT-proBNP), estimated glomerular filtration rate (eGFR), angiotensin-converting enzyme inhibitor (ACEi)/ angiotensin-receptor blocker (ARB) use, beta blocker use, and aldosterone antagonist use. Candidate covariates entered in the model were baseline characteristics with univariate all-cause mortality associations with p ≤0.1. Covariates that had a p > 0.1 but a theoretical basis for potential confounding were forced into the model. Models were built using backward elimination (likelihood ratio test) where all covariates with a p < 0.1 were retained.
Laboratory values from the Heart Failure Network (HFN) core lab were preferentially used and when missing, local site laboratory values were substituted. Glomerular filtration rate was estimated by the Modification of Diet in Renal Disease equation (23), as specified by the ROSE-AHF protocol. Statistical analysis was performed with IBM SPSS Statistics version 24 (IBM Corp., Armonk, NY), and statistical significance was defined as 2-tailed p < 0.05 for all analyses.
Results
Baseline characteristics for the 316 patients included in this analysis are presented in Table 1 and mirrored the overall ROSE-AHF population: patients tended to be white males with ischemic cardiomyopathy, multiple co-morbid conditions, and a high prevalence of physical exam findings consistent with volume overload. Given there were no significant differences in sodium excretion across the treatment groups in the ROSE-AHF trial (p = 0.75 for low-dose dopamine vs placebo; p = 0.52 for low-dose nesiritide vs placebo), we elected to analyze the groups together. The median eGFR for these patients was 43.7 mL/min/1.73m2 (IQR 32.5 – 55.4 mL/min/1.73m2). When stratified by natriuretic response, those with a positive sodium balance (who excreted less than the 2g) tended to have higher baseline NT-proBNP levels and a lower eGFR (Table 1). A considerable percentage of patients (28.5%) exhibited positive sodium balance through 24 hours (Table 1, Central Illustration), and this percentage was similar on Day 2 (29.7%) and Day 3 (32.4%). However, net positive fluid balance (n = 26, 8.2%) and weight gain (n = 50, 15.8%) occurred less commonly, and only seven (2.2%) participants exhibited both net fluid and weight gain. There was only a modest correlation between sodium excretion and weight loss (r = 0.53) and between sodium excretion and net fluid loss (r = 0.67) (Online Table 1).
Table 1.
Baseline Characteristics of the Study Participants According to Sodium Excretion on Day 1
| Characteristics | Study Cohort (N = 316) |
Positive Sodium Balance (n = 90) |
1-2g Na Excretion (n = 85) |
>2g Na Excretion (n = 141) |
p |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (y) | 71(63–80) | 70 (63–80) | 71 (63–83) | 71 (62–79) | 0.86 |
| Male | 73.7 | 72.2 | 68.2 | 7.0 | 0.25 |
| White | 76.3 | 71.1 | 76.5 | 79.4 | 0.37 |
| Past Medical History | |||||
| Ischemia as cause of HF | 58.2 | 58.9 | 56.5 | 58.9 | 0.93 |
| Hypertension | 84.2 | 84.4 | 82.4 | 85.1 | 0.86 |
| Diabetes Mellitus | 56.3 | 55.6 | 60 | 54.6 | 0.72 |
| Ejection Fraction (%) | 35.0 (22.0–54.0) | 35.5 (20.0–54.3) | 36.0 (23.0–55.0) | 33.0 (22.3–52.8) | 0.65 |
| Ejection Fraction (>50%) | 28.5 | 27.9 | 32.5 | 26.7 | 0.66 |
| Physical Exam | |||||
| Body Mass Index (kg/m2) | 31.0 (26.6–37.0) | 30.4 (25.7–37.0) | 31.3 (26.8–37.3) | 31.5 (27.0–36.3) | 0.71 |
| Rales | 57.2 | 58.4 | 56.6 | 56.7 | 0.66 |
| Jugular Venous Pressure (cm) | 95.4 | 94.3 | 96.4 | 95.4 | 0.43 |
| S3 Heart Sound | 24.6 | 22.7 | 22.4 | 27.2 | 0.64 |
| Peripheral Edema ≥ 2+ (4+ scale) | 71.4 | 70 | 69.4 | 73.6 | 0.29 |
| Systolic Blood Pressure, mmHg | 115 (104-127) | 114 (102-123.5) | 115 (105-126.5) | 116 (106-131.5) | 0.19 |
| Medications | |||||
| ACEi/ARB | 49.4 | 44.0 | 44.7 | 55.3 | 0.16 |
| β-blocker | 82.6 | 83.3 | 81.2 | 83.0 | 0.92 |
| Aldosterone antagonist | 28.5 | 28.9 | 29.4 | 27.7 | 0.96 |
| Furosemide Equivalents (mg) | 120 (60-180) | 120 (60-177) | 120 (60-170) | 120 (80-180) | 0.77 |
| Baseline Laboratory values | |||||
| Hemoglobin (g/dL) | 11.4 (10.3-12.6) | 11.6 (10.5-12.6) | 11.4 (10.3-12.5) | 11.3 (10.3-12.7) | 0.62 |
| Serum Sodium (mEq/L) | 139 (136-141) | 138 (134-141) | 138 (137-141) | 139 (137-141) | 0.77 |
| NT-proBNP, pg/mL | 5181 (2364–10349) | 7153 (3437–13052) | 4653 (1944–8497) | 5244 (2088–10553) | 0.02* |
| Creatinine, mg/dL | 1.7 (1.4–2.1) | 1.8 (1.5–2.3) | 1.7 (1.31–1.94) | 1.7 (1.4–2.0) | 0.06 |
| BUN, mg/dL | 37.0 (28.0–51.0) | 44.0 (28.5–60.0) | 34.0 (27.0–48.0) | 36.5 (28.0–48.9) | 0.12 |
| eGFR mL/min/1.73m2 | 43.7 (32.5–55.4) | 41.5 (28.3–49.6) | 44.8 (30.5–58.7) | 43.8 (34.7–56.1) | 0.03* |
Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; HF, heart failure; ICD, implantable cardioverter-defibrillator; NT-proBNP, N-terminal pro-B type natriuretic peptide. Continuous variables reported as median (IQR) and categorical variables are reported as percentage.
= p < 0.05
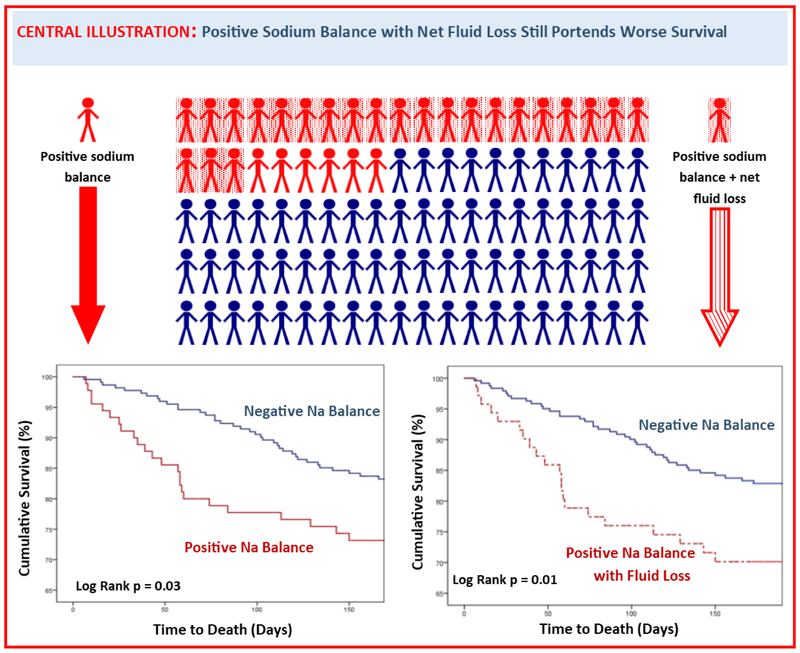
Central Illustration: Positive Sodium Balance with Net Fluid Loss Still Portends Worse Survival.
Urine sodium excretion, as metric of diuretic response, carries prognostic value. A considerable percentage (29%) of participants excreted less sodium than would be consumed in their diet (positive sodium balance), and these patients experienced decreased six-month survival (log rank p = 0.03). Even those who exhibited positive sodium balance and net fluid loss (23%) still experienced decreased survival (log rank p = 0.01).
Variability in Sodium Excretion with Diuresis
Sodium excretion through 24 hours was highly variable across patients (Figures 1A & 1B) despite aggressive diuretic dosing (median dose on day 1 = 200mg IV furosemide equivalents, IQR 100 – 280 mg). There was no overall correlation between sodium excretion and diuretic dose (r = 0.06, p = 0.27) and there was no difference in diuretic dose among those with positive sodium balance, intermediate response, and excellent response (p = 0.68). This lack of correlation between diuretic dose and sodium output persisted after controlling for eGFR (p =0.33). Within-patient analysis confirmed considerable variability in sodium excretion between hospital days with a modest correlation between day 1 and day 2 sodium excretion (r = 0.44, p < 0.001), but a weaker correlation between day 1 and day 3 (r = 0.18, p = 0.002, Online Table 2).
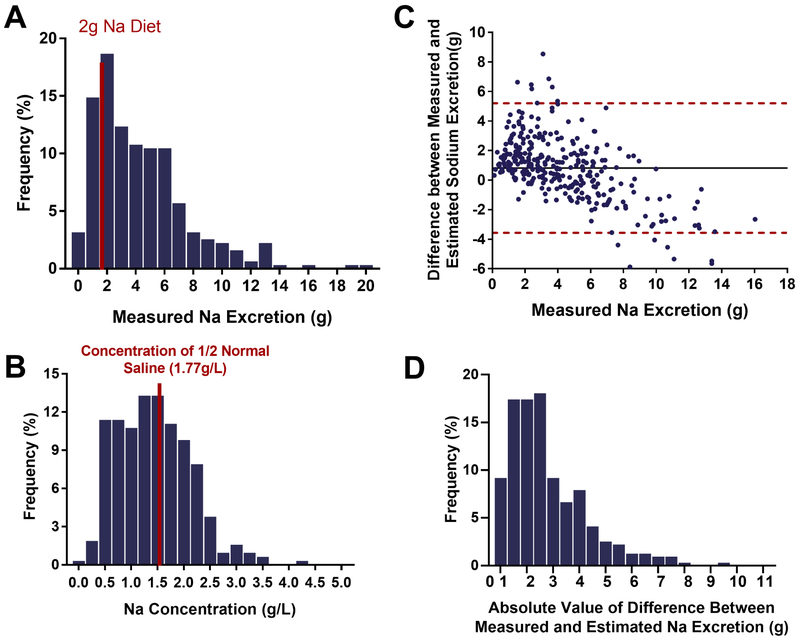
Figure 1: Variability in Sodium Excretion.
1A. Sodium excretion was highly variable across patients (Range: 0.12 – 19.8g, Median: 3.63g, IQR 1.85 – 6.02 g). Over a quarter of the patients (28.5%) exhibited positive sodium balance (indicated by the red bar) on the 2g/day diet despite aggressive diuresis. 1B. Urinary sodium concentration was also highly variable across patients (Range: 0.02 – 4.57g/L, Median: 0.69g/L, IQR 0.43 – 0.95g/L). 1C. Bland and Altman plot of differences between measured sodium excretion and estimated sodium excretion using the “half-normal saline” assumption. Mean of the differences, also known as the bias (black), and the limits of agreement (red) are reported. Mean of the differences: 0.8g. Mean of the absolute value of the differences: 1.8g. Lower limit of agreement: −3.6g Upper limit of agreement: 5.2g. 1D. Histogram of the absolute differences between measured sodium excretion and estimated sodium excretion using the “half-normal saline” assumption. Mean: 1.8g ± 1.6g.
Measured Sodium Excretion and the Assumption of a Consistent Urine Sodium Concentration
When the total urine output was multiplied by the concentration of half-normal saline, sodium excretion was overestimated in 69.9% of patients, and there were large under and overestimations at the individual level. The assumption overestimated by >1g in 47.1% of patients and by >2g in 24.3%, and it underestimated by >1g in 17.7% of patients and by >2g in 8.2% (Central Illustration). Figure 1C presents a Bland and Altman plot of estimated and measured absolute sodium excretion. Notably, the 95% limits of agreement were wide (−155.1 – 226.5 mmol or −3.6 – 5.2 g). The bias, or mean of the differences, indicated that the assumption overestimated by 35.7mmol (800mg), while the mean of the absolute value of the differences (Figure 1D) was considerably larger 78.2mmol (1.8g). Similarly, when comparing the estimated sodium concentration to the 77 mmol/L half-normal saline assumption there was poor agreement (Figure 1B).
Prognostic Value of the Various Metrics of Diuretic Response
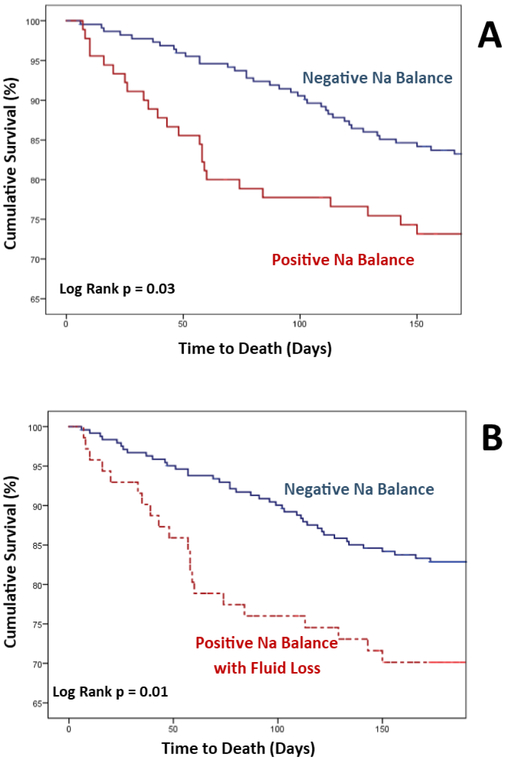
Sodium excretion was significantly associated with six-month all-cause mortality. For each doubling of sodium excretion, the odds of death were reduced by 20% in both univariate (HR = 0.80 per doubling of sodium excretion, 95% CI 0.66 – 0.95, p = 0.01) and multiple regression (HR = 0.80, 95% CI 0.65 – 0.97, p = 0.02) modeling. As sensitivity analyses (Online Table 3), the results using cumulative sodium excretion over the first 48 and 72 hours, respectively, were similar in both magnitude and direction (HR = 0.75, 95% CI 0.57-0.98, p = 0.04 for 48h excretion and HR = 0.74, 95% CI = 0.51-0.94, p = 0.02 for 72h excretion). However, total urine output (adjusted HR per L = 0.93, 95% CI 0.77 – 1.11, p = 0.43), net fluid balance (adjusted HR per L = 0.98, 95% CI 0.83 – 1.17, p = 0.87), and weight loss (adjusted HR per kg = 0.94, 95% CI 0.85 – 1.04, p = 0.25) were not significantly associated with survival. Positive sodium balance was associated with significantly decreased survival in Kaplan-Meier analysis (Central Illustration / Figure 2A), while net positive fluid balance (p = 0.57) and weight gain (p = 0.44) were not significantly associated with survival.
Figure 2. Natriuretic Response, Fluid Response, and Kaplan-Meier Plots of Cumulative Survival.
2A.Cumulative survival of patients stratified by sodium balance. Over a quarter (28.5%) of patients exhibited poor natriuretic response that would yield positive sodium balance on a 2g/day sodium diet. Kaplan-Meier analysis revealed these patients to have significantly decreased six-month survival (log rank p = 0.03). 2B. Cumulative survival of patients with discordant fluid and sodium response. Seventy-one patients experienced a discordant fluid and sodium response, defined as fluid loss in the setting of positive sodium balance. When compared against patients with negative sodium balance (≤ 2g/day), this group with a discordant response still showed a significantly decreased six-month survival in Kaplan-Meier analysis (log rank p = 0.01). The group of patients with positive sodium and fluid balance (N=7) was too small to allow for independent sub-analysis.
To further elucidate the relative importance of fluid vs. natriuretic responses, we examined patients who exhibited discordant responses. The 71 patients who exhibited both positive sodium balance and net fluid loss still experienced a significantly decreased six-month survival (Central Illustration / Figure 2B, p = 0.01). This association remained significant after controlling for baseline covariates and loop diuretic dose (HR = 2.02, 95% CI 1.17 – 3.46, p = 0.01).
Discussion
The primary findings of this study are: 1) Urinary sodium excretion during aggressive diuresis in ADHF patients with renal dysfunction is highly variable both across patients and within the same patient across different days; 2) As a consequence of such variability, the assumption of a consistent assumption for the salt content, such as “half-normal saline”, of loop diuretic induced urine does not accurately predict 24-hour sodium excretion; 3) Sodium excretion, but not metrics of fluid or weight response, carries significant prognostic value; 4) Even in the context of high diuretic dosing and documented net fluid loss, a positive sodium balance is relatively common and associated with significantly worse survival in ADHF patients. In conjunction with the well-established central pathophysiologic role of sodium in volume retention and its treatment, the current observations reinforce that sodium excretion is a critical variable in the treatment of ADHF patients and requires additional study.
Despite recognition of their shortcomings, fluid and weight assessments remain the standard quantitative metrics guiding treatment for heart failure in clinical practice, as well as endpoints in clinical trials (10,13,24). Notably, although essentially measuring the same underlying parameter, net fluid balance and weight loss correlate poorly, even in the setting of rigorous NIH-funded prospective trials on diuretic therapy (25). This is likely due in part to the logistical challenges of accounting for every milliliter of a patient’s fluid intake. Even when providers officially place patients on fluid restriction, nearly every hospital room has a faucet. Conversely, there is not such a ubiquitous and easily accessible source of sodium in hospitals.
Furthermore, it is not clear that change in body weight performs any better than measuring fluid intake and output. Patients may be weighed using different types of scales (bed, standing, sling), scales with different calibrations, and at different times of the day relative to meals, voids, or bowel movements. The variability of bowel movements is of even greater significance in patients undergoing fluid restriction, which has been linked to constipation (26). Finally, important nuances such as whether the patient’s telemetry box or shoes were included in the measurement are often overlooked. Sodium, on the other hand, is not affected by these factors.
The results of this analysis are consistent with the primacy of sodium in the pathophysiology of heart failure. Given the predominance of semipermeable membranes which allow water, but not ions, to move freely, fluid loss is a secondary effect due to sodium excretion. As highlighted by our analysis, urinary sodium concentration is highly variable, thus uncoupling fluid excretion from sodium excretion. By measuring only fluid excretion without urinary sodium or urinary sodium concentration, patients with lower urinary sodium concentrations are not always assessed as poor responders, as long as their urine output is deemed adequate. We would argue that there is an important difference between a patient who produces 2L of urine with a low urinary sodium concentration, and another patient who produced the identical amount of urine with a higher sodium content. This difference is captured when measuring total urinary sodium excretion, but not with strictly fluid metrics. However, it has yet to be demonstrated that routine performance of 24-hour urine collection to measure sodium excretion is feasible outside of a clinical trial setting.
To address this issue, our group has developed a sodium prediction equation to forecast sodium excretion following a bolus dose of loop diuretic (27). Much in the way that 24-hour creatinine collections have largely been supplanted by eGFR derived from an equation and a spot serum creatinine value, we have developed a formula that can estimate the amount of sodium excretion from a dose of loop diuretic using a spot urine sodium and creatinine level. We are currently in the process of validating this equation in a larger cohort (ClinicalTrials.gov Identifier: NCT02546583). Our current analysis provides justification for a shift in emphasis away from fluid-based metrics toward sodium-based metrics, and the forthcoming tool may help to inform therapy for these complex patients. One strategy would be to titrate loop diuretic dose to measured sodium excretion, instead of solely utilizing fluid and weight metrics to assess response.
There were several limitations that should be acknowledged. Importantly, this was a post-hoc analysis of the ROSE-AHF trial, which was not designed to compare different metrics of monitoring diuresis. As such, these results should be considered hypothesis-generating. The ROSE cohort was primarily comprised of mostly white males, and by design, all patients had an eGFR less than 60 mL/min/1.73m2. As a result, it is unclear how the results will apply to the broader ADHF population. In addition, although the 24-hour urine collection is the gold standard to evaluate sodium balance, such a collection is by definition comprised of all urine produced in a day. This limits inferences made specifically about the composition urine produced solely as the result of the loop diuretic. Although patients were placed on a 2g sodium diet, individuals may have actually consumed more or less than 2g, limiting the accuracy of assumptions made regarding sodium balance.
Conclusion:
In line with the central role of sodium in the pathophysiology of ADHF and its treatment, sodium excretion was more strongly associated with six-month survival than either net fluid balance or weight change. However, the sodium content of urine in ADHF patients was highly variable, indicating that an assumption of a consistent urine sodium concentration is inadequate, and urine sodium content needs to be determined with each diuretic dose for each individual patient. Additional research is needed to better understand the role of urine sodium content in personalizing ADHF therapy.
Supplementary Material
Core Clinical Competencies and Translational Outlook Implications.
COMPETENCY IN MEDICAL KNOWLEDGE: In patients with ADHF receiving high-dose loop diuretics, sodium excretion is highly variable. Higher degrees of sodium excretion are associated with improved survival, while the traditional fluid-based metrics, such as weight change and net fluid balance, were not. Patients with a positive sodium balance, even when accompanied by a net negative fluid balance, had worse survival compared with all patients who had a net negative sodium balance.
TRANSLATIONAL OUTLOOK: Direct measurement of urinary sodium excretion may provide a viable alternative to urine output and fluid balance to guide diuretic therapy in ADHF. Additional research is required to better understand how measurement of urinary sodium content can guide heart failure management.
Acknowledgments
Funding: This work was supported by NIH Grants, K23HL114868, L30HL115790, R01HL139629, R21HL143092, R01HL128973 (JT), and K23DK097201 (FPW).
Abbreviations.
- ACEi
angiotensin-converting enzyme inhibitor
- ADHF
Acute Decompensated Heart Failure
- ARB
angiotensin-receptor blocker
- BioLINCC
Biologic Specimen and Data Repository Information Coordinating Center
- eGFR
Estimated Glomerular Filtration Rate
- HFN
Heart Failure Network
- LVEF
Left Ventricular Ejection Fraction
- NT-proBNP
N-terminal pro-B natriuretic peptide (NT-proBNP)
- ROSE-AHF
Renal Optimization Strategies Evaluation in Acute Heart Failure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: All authors have no relevant disclosures
References
- 1.Cadnapaphornchai MA, Gurevich AK, Weinberger HD, Schrier RW. Pathophysiology of sodium and water retention in heart failure. Cardiology 2001;96:122–31. [DOI] [PubMed] [Google Scholar]
- 2.Mullens W, Verbrugge FH, Nijst P, Tang WHW. Renal sodium avidity in heart failure: from pathophysiology to treatment strategies. Eur Heart J 2017;38:1872–1882. [DOI] [PubMed] [Google Scholar]
- 3.Nijst P, Verbrugge FH, Grieten L et al. The pathophysiological role of interstitial sodium in heart failure. J Am Coll Cardiol 2015;65:378–388. [DOI] [PubMed] [Google Scholar]
- 4.Gheorghiade M, Pang PS. Acute heart failure syndromes. J Am Coll Cardiol 2009;53:557–73. [DOI] [PubMed] [Google Scholar]
- 5.Ponikowski P, Voors AA, Anker SD et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 6.Writing Committee M, Yancy CW, Jessup M et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013;128:e240–327. [DOI] [PubMed] [Google Scholar]
- 7.Kociol RD, McNulty SE, Hernandez AF et al. Markers of decongestion, dyspnea relief, and clinical outcomes among patients hospitalized with acute heart failure. Circ Heart Fail 2013;6:240–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vargo DL, Kramer WG, Black PK, Smith WB, Serpas T, Brater DC. Bioavailability, pharmacokinetics, and pharmacodynamics of torsemide and furosemide in patients with congestive heart failure. Clin Pharmacol Ther 1995;57:601–9. [DOI] [PubMed] [Google Scholar]
- 9.Blair JE, Khan S, Konstam MA et al. Weight changes after hospitalization for worsening heart failure and subsequent re-hospitalization and mortality in the EVEREST trial. Eur Heart J 2009;30:1666–73. [DOI] [PubMed] [Google Scholar]
- 10.Bart BA, Goldsmith SR, Lee KL et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 2012;367:2296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gheorghiade M, Konstam MA, Burnett JC Jr. et al. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials. JAMA 2007;297:1332–43. [DOI] [PubMed] [Google Scholar]
- 12.Konstam MA, Gheorghiade M, Burnett JC Jr. et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA 2007;297:1319–31. [DOI] [PubMed] [Google Scholar]
- 13.Felker GM, Lee KL, Bull DA et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 2011;364:797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Binanay C, Califf RM, Hasselblad V et al. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA 2005;294:1625–33. [DOI] [PubMed] [Google Scholar]
- 15.Adrogue HJ, Madias NE. Hypernatremia. N Engl J Med 2000;342:1493–9. [DOI] [PubMed] [Google Scholar]
- 16.Yee J, Parasuraman R, Narins RG. Selective review of key perioperative renal-electrolyte disturbances in chronic renal failure patients. Chest 1999;115:149S–157S. [DOI] [PubMed] [Google Scholar]
- 17.Marenzi G, Lauri G, Assanelli E et al. Serum to urinary sodium concentration ratio is an estimate of plasma renin activity in congestive heart failure. European Journal of Heart Failure 2002;4:597–603. [DOI] [PubMed] [Google Scholar]
- 18.Verbrugge FH, Nijst P, Dupont M, Penders J, Tang WH, Mullens W. Urinary composition during decongestive treatment in heart failure with reduced ejection fraction. Circ Heart Fail 2014;7:766–72. [DOI] [PubMed] [Google Scholar]
- 19.Zazzeron L, Ottolina D, Scotti E et al. Real-time urinary electrolyte monitoring after furosemide administration in surgical ICU patients with normal renal function. Ann Intensive Care 2016;6:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen HH, AbouEzzeddine OF, Anstrom KJ et al. Targeting the Kidney in Acute Heart Failure: Can Old Drugs Provide New Benefit? Renal Optimization Strategies Evaluation in Acute Heart Failure (ROSE AHF) Trial. Circ-Heart Fail 2013;6:1087–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen HH, Anstrom KJ, Givertz MM et al. Low-Dose Dopamine or Low-Dose Nesiritide in Acute Heart Failure With Renal Dysfunction The ROSE Acute Heart Failure Randomized Trial. Jama-J Am Med Assoc 2013;310:2533–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jain AK, Chen HH. ROSE-AHF and lessons learned. Curr Heart Fail Rep 2014;11:260–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stevens LA, Coresh J, Feldman HI et al. Evaluation of the modification of diet in renal disease study equation in a large diverse population. J Am Soc Nephrol 2007;18:2749–57. [DOI] [PubMed] [Google Scholar]
- 24.Costanzo MR, Guglin ME, Saltzberg MT et al. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol 2007;49:675–83. [DOI] [PubMed] [Google Scholar]
- 25.Testani JM, Brisco MA, Kociol RD et al. Substantial Discrepancy Between Fluid and Weight Loss During Acute Decompensated Heart Failure Treatment. Am J Med 2015;128:776–83 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anti M, Pignataro G, Armuzzi A et al. Water supplementation enhances the effect of high-fiber diet on stool frequency and laxative consumption in adult patients with functional constipation. Hepatogastroenterology 1998;45:727–32. [PubMed] [Google Scholar]
- 27.Testani JM, Hanberg JS, Cheng S et al. Rapid and Highly Accurate Prediction of Poor Loop Diuretic Natriuretic Response in Patients With Heart Failure. Circ Heart Fail 2016;9:e002370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.