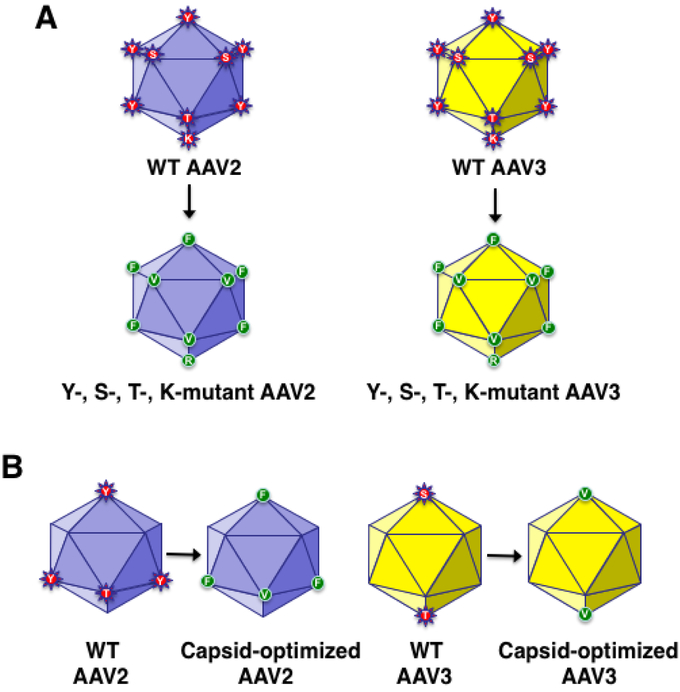
FIGURE 2: Schematic representation of capsid-modified next generation of recombinant AAV vectors.
Surface-exposed, specific tyrosine (Y), serine (S), and threonine (T) residues on AAV capsids can be phosphorylated, which is a signal for ubiquitination. Surface-exposed, specific lysine (K) residues on AAV capsids can be ubiquitinated, and subsequently degraded by the host cell proteasome machinery. Site-directed mutagenesis of these residues leads to the generation of AAV vectors that are more efficient at reduced vector doses, and consequently, less immunogenic. Specific examples of the most efficient AAV2 [55-57,59,61], and AAV3 [42,43,47,61,63], serotype vectors generated thus far, are also depicted. AAV2 data from Zhong L, Li B, Mah CS, et al.. Next generation of adeno-associated virus 2 vectors: Point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc Natl Acad Sci USA 2008;105:7827-32, Markusic DM, Herzog RW, Aslanidi GV, et al.. High-efficiency transduction and correction of murine hemophilia B using AAV2 vectors devoid of multiple surface-exposed tyrosines. Mol Ther 2010;18:2048-56, Martino AT, Basner-Tschakarjan E, Markusic DM, et al.. Engineered AAV vector minimizes in vivo targeting of transduced hepatocytes by capsid-specific CD8+ T cells. Blood 2013;121:2224-33, Aslanidi GV, Rivers AE, Ortiz L, et al.. Optimization of the capsid of recombinant adeno-associated virus 2 (AAV2) vectors: The final threshold? PLoS One 2013;8:e59142, and Ling C, Li B, Ma W, et al.. Development of optimized AAV serotype vectors for high-efficiency transduction at further reduced doses. Hum Gene Ther Meth 2016;27:143-9, with permission. AAV3 data from Cheng B, Ling C, Dai Y, et al.. Development of optimized AAV3 serotype vectors: Mechanism of high-efficiency transduction of human liver cancer cells. Gene Ther 2012; 19:375-84, Ling C, Wang Y, Zhang Y, Ejjigani A, Yin Z, Lu Y, Wang L, Wang M, Li J, Hu Z, Aslanidi GV et al.: Selective in vivo targeting of human liver tumors by optimized AAV3 vectors in a murine xenograft model. Hum Gene Ther (2014) 25(12):1023-1034, Li S, Ling C, Zhong L, Li M, Su Q, He R, Tang Q, Greiner DL, Shultz LD, Brehm MA, Flotte TR et al.: Efficient and targeted transduction of nonhuman primate liver with systemically delivered optimized AAV3b vectors. Mol Ther (2015) 23(12):1867-1876, Ling C, Li B, Ma W, et al.. Development of optimized AAV serotype vectors for high-efficiency transduction at further reduced doses. Hum Gene Ther Meth 2016;27:143-9, and Vercauteren K, Hoffman BE, Zolotukhin I, et al.. Superior in vivo transduction of human hepatocytes using engineered AAV3 capsid. Mol Ther 2016;24:1042-49 with permission.