Table 5.
Hydroacylation Scope with α-Aryl Aldehydes[a]
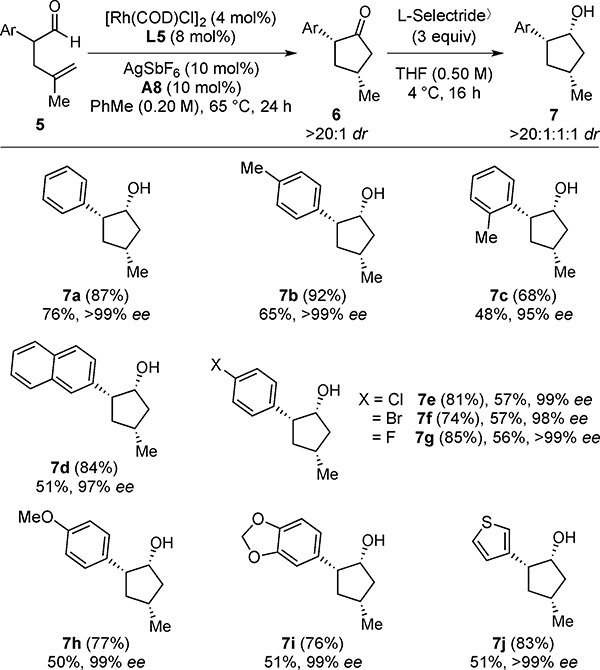 |
With 0.10 mmol of 5.
1H NMR yields of 6 are given in parentheses. Isolated yields over two steps are given of 7. Diastereoselectivities of each step were determined by 1H NMR analysis of the unpurified reaction mixture. Enantioselectivities (ee’s) were determined by chiral SFC analysis.
