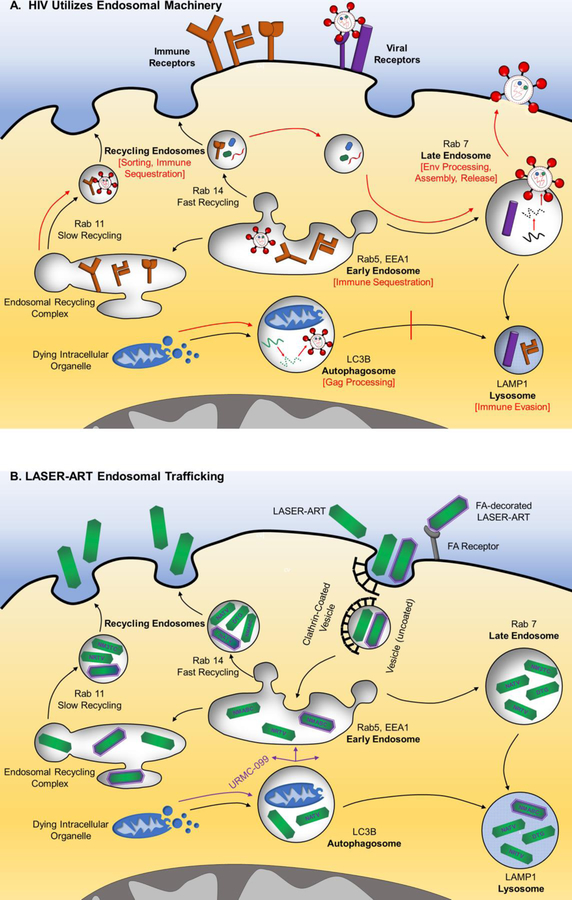
Fig 1.
Utilization of endocytic machinery in (A) HIV Infection: Endocytosis and autophagy direct extracellular materials or dying organelles to the lysosome, respectively (black arrows). HIV binds CD4 and CCR5 or CXCR4 to enter host cells. Provirus resides in early, recycling, and late endosomes in addition to within autophagosomes. HIV manipulates subcellular organelle trafficking to its advantage (red arrows). Early endosomes sequester MHC-I and immune checkpoint inhibitor, CTLA-4. Recycling endosomes aid in sorting proviral proteins prior to assembly, as well as in sequestration of CTLA-4 and co-stimulatory molecules, CD80/86. HIV promotes autophagosome formation, where Gag polyprotein processing occurs, and inhibits its own degradation in the lysosome. Late endosomes assist in envelope protein processing, proviral assembly in MP, and viral release. HIV evades adaptive immunity by degrading CD4 and MHC-I in the lysosome. (B) LASER-ART delivery is shown. Nanoformulated nanocrystals accumulate in high concentrations within recycling and late endosomes and to lesser levels in early endosomes or lysosomes. URMC-099, a mixed lineage kinase-3 inhibitor, enhances shuttling of antiretroviral particles from the autophagosome to each of these compartments, where they inhibit key HIV processes due to their proximity to virus. Recycling endosomes also provide for sustained release of free-drug from nanocrystals present in MP. N- nanoformulated; M- myristoylated; FA- folic acid; 3TC- lamivudine; ABC- abacavir; ATV- atazanavir; DTG- dolutegravir encased in europium doped cobalt-ferrite; RTV- ritonavir.