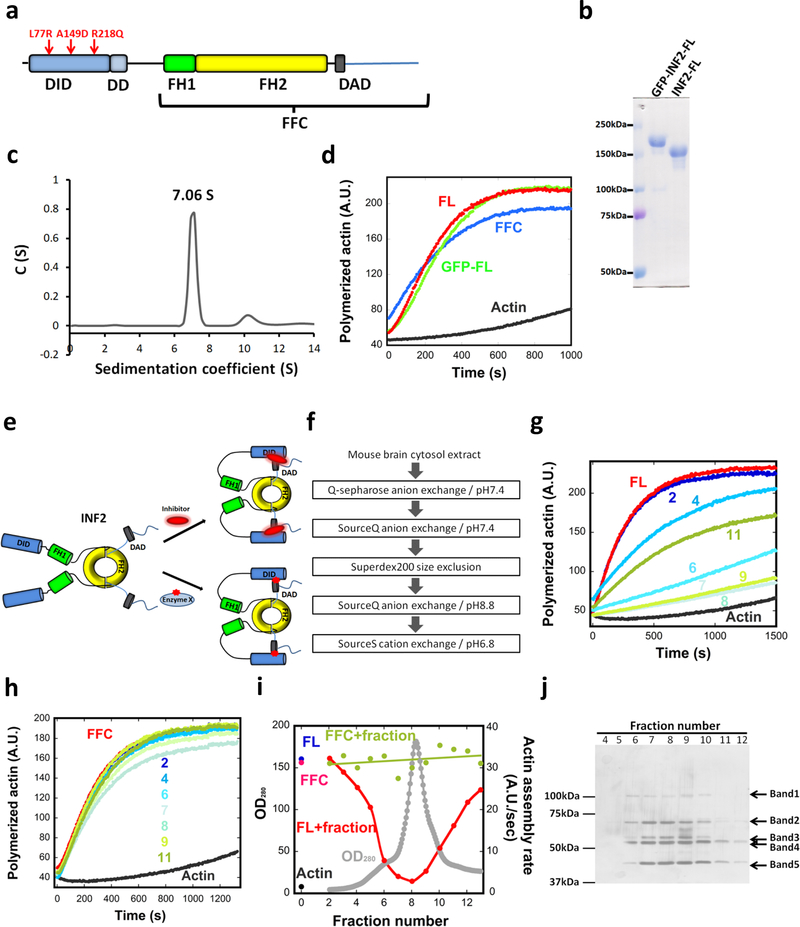
Figure 1. Constitutive activity of purified INF2, and inhibition by a brain factor.
(a) Schematic diagram of human INF2-nonCAAX (1240 residues). The A149D mutation disrupts DID/DAD interaction. The R218Q and L77R link to FGSG and CMTD, respectively. Domain boundaries: DID, 32–261; DD, 263–342; FH1, 421–520; FH2, 554–940; DAD, 971–1000. FFC construct: 469–1240. (b) Coomassie-stained SDS-PAGE of purified human INF2-nonCAAX, with and without the GFP-Strep tag. Experiment performed five times with similar results. (c) Sedimentation velocity analytical ultracentrifugation of INF2-nonCAAX (3.8 μM). Mass: 270.3 kDa (sedimentation), 134.6 kDa (sequence). Experiment performed once. (d) Pyrene actin polymerization assay (2μM actin) using 20nM INF2 (with or without GFP-Strep) or INF2 FFC. Experiment performed six times with similar results. (e) Schematic of two possible mechanisms for facilitated autoinhibition of INF2. Upper: direct inhibitor binding. Lower: INF2 post-translational modification (red star). (f) Flow chart of chromatography steps for inhibitor purification. (g) and (h) Representative pyrene-actin assays (2μM actin) containing 20nM INF2-FL (g) or 20nM INF2-FFC (h) with or without indicated BIF fractions (fractions 2–11, from Fig. 2i). Experiment performed once. (i) Graph showing protein elution profile from the final SourceS column (OD280 nm, gray) as well as activities of 20 nM INF2-FL (red) or FFC (green) in pyrene-actin assays on addition of the eluted fraction. Activity of INF2-FL alone (blue), INF2 FFC alone (magenta) and actin alone (black) shown on left side. Experiment performed once. (j) Silver stained SDS-PAGE of indicated fractions from figure 2i. Bands identified as follows: Band1, Exportin1/7; Band 2, VATA and HSP7C; Band 3, CAP1, CAP2, VATB2; Band 4, CAP1, CAP2, VATA; and Band 5, actin. Experiment performed once.