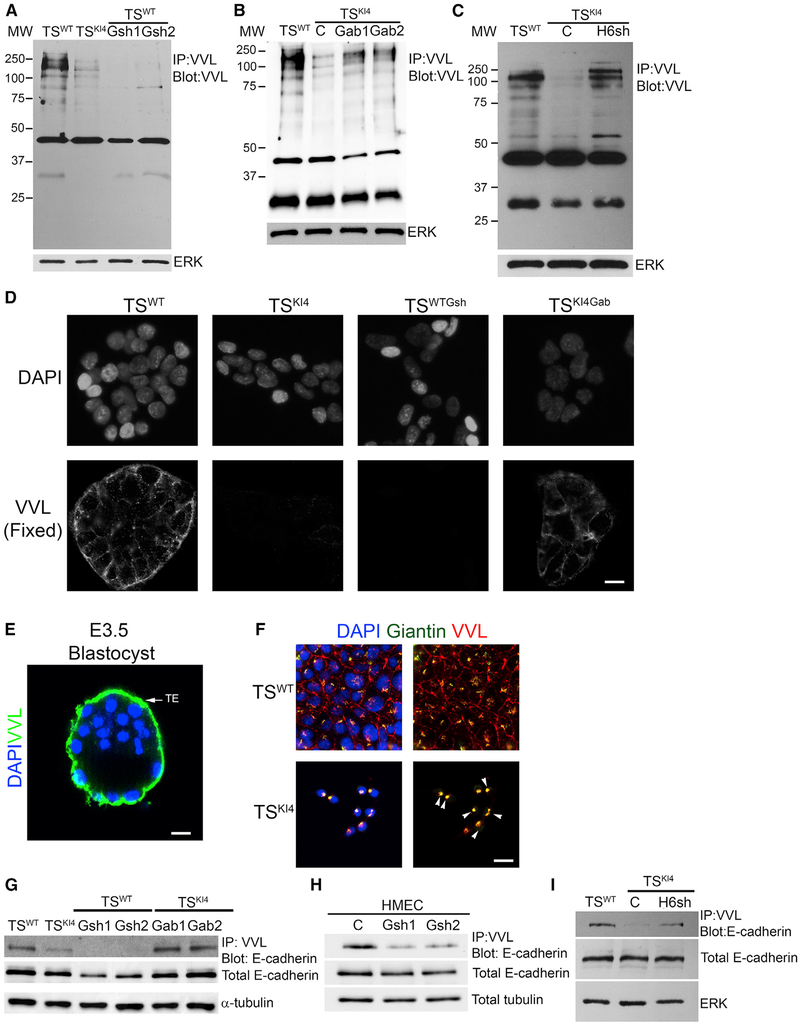
Figure 7. Reduced O-GalNAc Glycosylation in Cells Lacking GALNT3 that Is Restored by Re-expression of GALNT3.
(A) Total protein O-GalNAc glycosylation is reduced with the loss of GALNT3 in TSWTGsh1 and TSWTGsh2 cells. IP with VVL agarose and blotting with biotinylated VVL are shown. Western blots of TSWT and TSKI4 cells expressing control shRNA (C) or TSWT cells expressing two independent Galnt3 shRNAs (TSWTGsh1 and TSWTGsh2) are representative of three independent experiments.
(B) Re-expression of GALNT3 in TSKI4 cells (TSKI4Gab1 and TSKI4Gab2) increases protein O-GalNAc glycosylation. Experiments were performed as in (A). Western blots of TSWT and TSKI4 cells expressing a control lentiviral vector (C) or or TSKI4 cells infected with a lentiviral vector expressing human Galnt3 (TSKI4Gab1 and TSKI4Gab2) are representative of three independent experiments.
(C) Knockdown of HDAC6 in TSKI4 cells (TSKI4H6sh) increases total protein O-GalNAc glycosylation similar to TSWT cells. Experiments were performed as in (A). Western blots of TSWT cells and TSKI4 cells expressing control shRNA (C) or TSKI4 cells expressing HDAC6 shRNA (TSKI4H6sh) are representative of two independent experiments.
(D) Absence of cell surface O-GalNAc glycosylation in TSKI4 and TSWTGsh cells with reduced GALNT3. VVL immunofluorescence staining in non-permeabilized TSWT and TSKI4 cells infected with a control virus, TSWT cells infected with Galnt3 shRNA (TSWTGsh), and TSKI4 cells re-expressing GALNT3 (TSKI4Gab) are shown. Images are representative of three independent experiments.
(E) O-GalNAc glycosylation of the trophoectoderm (TE) of intact wild-type E3.5 hatched blastocyst is shown by confocal microscopy using biotinylated VVL. Image is representative of seven blastocysts. DAPI (blue) and VVL (green).
(F) Reduced GALNT3 expression in TSKI4 cells induces retention of O-GalNAc glycosylated proteins in the Golgi. DAPI (blue), VVL (red), and Giantin (Golgi) (green) staining of Triton-permeabilized cells. Arrowheads show co-localization between VVL and Giantin in the Golgi. Images are representative of three independent experiments.
(G and H) O-GalNAc glycosylation of E-cadherin is reduced in cells lacking GALNT3. IP with VVL agarose and blotting with anti-E-cadherin antibody are shown for TS cells (G) and HMECs (H). Western blots are representative of three independent experiments.
(I) Knockdown of HDAC6 increases E-cadherin O-GalNAc glycosylation in TSKI4H6sh cells. Experiments were performed as in (G and H). Western blots are representative of two independent experiments.
IP, immunoprecipitation; MW, molecular weight in kDa; white bar represents 50 μm.
See also Figures S6 and S7.