Abstract
The nasal valve area has the minimal cross-sectional area of the upper airways. Obstructive sleep apnea (OSA) is a common disorder. It has been reported that nasal obstruction may be associated with OSA. The aim of this study was to investigate whether the use an internal nasal dilator may be able to affect respiratory pattern in a group of patients with OSA. The use of internal nasal dilator was able to significantly reduce two relevant respiratory outcomes, such as the apnea-hypopnea index and the oxygen desaturation index, notably there was also a positive trend for the reduction of total sleep time with HbO2 <90%). Nas-air® was also able to significantly improve restorative sleep performance. In conclusion, the present study demonstrates that Nas-air® is a new internal nasal dilator potentially capable to significantly improve respiratory outcomes and sleep quality. (www.actabiomedica.it)
Keywords: nasal valve, internal nasal dilator, Nas-air ®, obstructive sleep apnea, respiratory parameters
Introduction
Obstructive sleep apnea (OSA) is a serious, potentially life-threatening disorder characterized by recurrent episodes of upper-airway collapse during sleep. The intermittent partial or complete occlusion of the upper airway (termed hypopneas and apnea, respectively), due to a combination of excess tissue and inappropriate upper airway muscle relaxation, often leads to hypoxemia and hypercapnia (1). Symptomatic OSA is common, and the disease prevalence is higher in different population subsets, including overweight or obese people, and older individuals (2). In fact, OSA affects 5% to 10% of middle-aged adults and up to 20% of adults over 65 years of age (3).
Sleep apnea has been associated with many health-related illnesses ranging from cognitive impairment, memory loss, depression, metabolic disorders, and, most seriously, cardiovascular diseases, such as ischemic heart disease, stroke, and chronic heart failure (4). Notably, the prevalence of adverse outcomes is typically dose-dependent: an increased number of apneas and hypopneas per hour of sleep is reflected in the apnea/hypopnea index (AHI), and the severity of oxygen desaturation (5).
Typical treatments for patients with OSA include continuous positive airway pressure (CPAP) therapy, oral appliances (those that advance the mandible and those that prevent relapse of the tongue), various surgeries that modify the upper airway, and/or weight loss (dietary, pharmacologically, and surgically induced).
Actually, CPAP is considered the “standard of care” for OSA treatment, as the therapeutic use of CPAP is able to significantly improve many of the acute pathophysiologic responses that result from sleep-disordered breathing (6). However, despite these relevant benefits, the therapeutic acceptance, compliance, and adherence remain significant challenges to patients and clinicians. Indeed, the real adherence to CPAP therapy averages about 50%, ranging from 30 to 70% (7). Therefore, many efforts are tried to improve this problem and/or to use other ways. In this regard, nasal obstruction is a common problem as reported by almost 20% of the general population, and about one-third of the sleep apnea patients (8). Significantly, OSA patients with nasal obstruction are more likely to suffer from daytime sleepiness and to have impaired quality of life than other OSA patients.
Interestingly, the anterior portion of the nasal cavities, from the nostrils to the nasal valve, is the region of the greatest nasal airflow resistance and where there is the narrowest segments of the nasal cavity (9). Therefore, this segment is very important for the nasal physiology and the main nasal symptom: obstruction. The relevance of nasal anatomy assessment has been deeply investigated in OSA patients by Leitzen and colleagues (10). They concluded that a careful nasal examination, clinical and functional, should be performed in all OSA patients. Consequently, some studies aimed to investigate whether nasal dilation could be useful in OSA patients. Colrain and colleagues studied an intranasal device, consisting of a small valve inserted into each nostril, in 32 OSA patients (11). The apnea-hypopnea index (AHI) and oxygen desaturation index (O2DI), and snoring score significantly decreased after using this device. This interesting outcome was partially confirmed by another study that investigated an internal nasal dilator (Nozovent) as some patients were responders to it and snoring was significantly diminished (12). Further, McLean and colleagues evaluated an external dilator strip (Breathe Right) in 10 patients with OSA and nasal obstruction (13). They reported that dilating the nose reduced mouth breathing during sleep and OSA severity. However, these outcomes were conflicting with a previous study that demonstrated no effect of nasal device on snoring and quality of sleep (14). On the other hand, it has been recently reported that an internal nasal dilator (Nas-air®) was able to significantly reduce snoring score (15). As this issue is controversial, we performed a study in a group of OSA patients with the aim to demonstrate the effectiveness of Nas-air® on respiratory pattern.
Materials and Methods
The present cross-sectional study included 19 inpatients with OSA diagnosis.
Inclusion criteria were: adult age and OSA diagnosis according to validated criteria (16). Exclusion criteria were: anatomical clinically relevant problems (e.g. very severe septal deviation and/or turbinate hypertrophy, such as grade IV), disorders and current medications potentially able to interfere with findings.
The patients were visited and undergone otorhinolaryngological visit, including anterior rhinoscopy. During the otorhinolaryngological visit, the following parameters were considered: age, gender, body mass index (BMI); a fibro-endoscopy was also performed.
Subjective parameters were evaluated by the patients, and include perception of nasal obstruction, sleep quality, and olfaction; they were measured by a visual analogue scale (VAS). VAS score for nasal obstruction ranged from 0 (=completely blocked nose) to 10 (=completely patent nose); VAS score for olfaction ranged from 0 (=no smell) to 10 (=optimal smell); VAS score for quality of sleep ranged from 0 (=worst sleeping) to 10 (optimal sleeping). In addition, VAS was used for assessing the satisfaction for the Nas-Air® (0=bad; 10=best).
Daytime sleepiness was evaluated with the Epworth Sleepiness Scale (ESS): an ESS score of ≥10 was considered excessive daytime sleepiness (17). In addition, the STOP-Bang (18), the Restorative Sleep (19) questionnaires, and Mallampati scale (20) were used.
Cardiorespiratory nocturnal monitoring was performed in all patients and was done in ambient air and spontaneous breathing using a portable 4-channel/8-track polygraph (WristOx2, Nonin, the Netherlands). Oxyhemoglobin saturation, heart rate, body posture, oral-nasal air flow, snoring sounds, and thoracic and abdominal movements were recorded in detail. AHI (apnea-hypopnea index), ODI (oxygen desaturation index), TST90 (total sleep time with oxyhemoglobin saturation below 90%), SaO2-Nadir % and Restoring Sleep were calculated
The Nas-air® (E.P. Medica, Fusignano, Italy) was given with appropriate instruction for the use, such as the internal nasal dilator should be applied into the nose at bedtime. All patients signed an informed consent to participate in the study. Patients were evaluated the first night (without any device) and the second one (with Nas-air®).
Clinical characteristics were reported as mean ± standard deviation (SD) for continuous variables and as percentage for categorial variables. The normal distribution of continuous variables was verified. Continuous parameters were analyzed by Student’s T-test for paired samples. Significance values assumed for p <0.005 All the analysis have been conducted with SPSS 21 software.
Results
The present study included 19 patients (4 females, 5 males, mean age 61±13.5 years) suffering from severe OSA with mean AHI 38.7±30.8. Mean BMI was 32.4±6.7; mean neck circumference 41.3±2.2).
Table 1 shows clinical characteristics of the patients in detail.
Table 1.
Clinical characteristics of the OSA patients. Data are expressed ad mean±SD or absolute number (and percentage).
| Population (n=19) | |
| Mean age | 61.0±13.5 |
| Smokers n (%) | 4 (21.1) |
| Gender females | 4 (21.1) |
| BMI | 32.4±6.7 |
| Sleepiness n (%) | 15 (78.9) |
| Neck circumference | 41.3±2.2 |
| Weakness n (%) | 5 (45.5) |
| Sleep hours | 6.6±1.1 |
| ESS | 6.5±3.8 |
| STOP BANG | 5.4±1.5 |
| MALLAMPATI | |
| 0 | 0 (0) |
| 1 | 0 (0) |
| 2 | 1 (5.3) |
| 3 | 6 (31.6) |
| 4 | 12 (63.2) |
| Turbinate hypertrophy | |
| 0 | 1 (5.3) |
| 1 | 8 (42.1) |
| 2 | 10 (52.6) |
| Bilaterally compromised nasal valve n (%) | 11 (57.9) |
| VAS nasal obstruction | 6.7±1.9 |
| VAS sleep quality | 4.4±2.3 |
| VAS smell | 7.8±2.9 |
| PO2 | 79.3 ±11.1 |
| PCO2 | 41.6±4.6 |
| pH | 7.42±0.03 |
| HCO3 | 27.0± 2.6 |
| SaO2 Mean | 95.3± 2.3 |
| HR bpm Mean±SD | 78.6±10.2 |
| Frequent awakes n (%) | 4 (21.1) |
| Chocking n (%) | 3 (15.8) |
| Reported Apnea n (%) | 15 (78.9) |
| Snoring n (%) | 16 (84.2) |
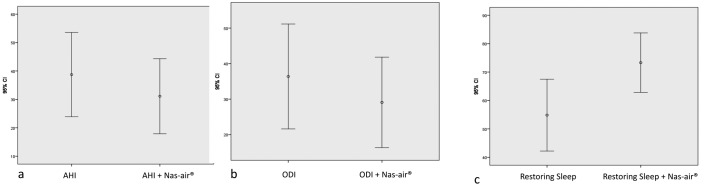
Table 2 shows the principal polygraphic parameters without and with the use of Nas-air® in the OSA patients. The use of Nas-air® significantly reduced AHI values (38.7±30 vs 31.1±27.4; p=0.000) and ODI scores (36.4±30.6 vs 29.0±26.4; p=0.001) as shown in Figure 1. In addition, the use of Nas-air® significantly increased the restoring sleep score (54.8±26.2 vs 73.3±21.7; p=0.000).
Table 2.
Comparison of polygraphic parameters without and with NasAir®
| Without NasAir® | With NasAir® | P-value | |
| AHI Mean±SD | 38.7±30.8 | 31.1±27.4 | 0.000 |
| ODI events/h Mean±SD | 36.4±30.6 | 29.0±26.4 | 0.001 |
| TST90 Mean±SD | 27.6±31.3 | 19.7±25.0 | 0.055 |
| Restoring Sleep Mean±SD | 54.8±26.2 | 73.3±21.7 | 0.000 |
| SaO2 % Nocturnal Mean±SD | 91.0±3.6 | 92.1±3.0 | 0.052 |
| SaO2-Nadir % Mean±SD | 75.1±11.3 | 76.2±10.6 | 0.588 |
Figure 1.
a=AHI values without and with Nas-air®; b=ODI scores without and with Nas-air®; c=Restoring sleep without and with Nas-air®
Moreover, there was a favorable trend for the use of Nas-air® concerning TST90, nocturnal SaO2 and Nadir-SaO2 as shown in Table 2.
Discussion
OSA is a breathing disorder characterized by narrowing of the upper airway that impairs normal ventilation during sleep. Recent reviews on the evaluation and management of CSA and sleep-related hypoventilation have been published as recently discussed (21). The clinical relevance of OSA depends on the large impact on the general population (22).
The consequences of untreated OSA are wide and may significantly vary, in fact, it has been postulated that they result from the fragmented sleep, intermittent hypoxia, and hypercapnia, intrathoracic pressure swings, and increased sympathetic nervous activity that accompanies disordered breathing during sleep. Individuals with OSA often feel unrested, fatigued, and sleepy during the daytime. They may suffer also rom impairments in vigilance, concentration, cognitive function, social interactions, and quality of life. Unfortunately, these declines in daytime function can translate into higher rates of job-related and motor vehicle accidents. Moreover, patients with untreated OSA may be at increased risk of developing cardiovascular disease, including difficult-to-control blood pressure, coronary artery disease, congestive heart failure, arrhythmias and stroke (23). OSA is also associated with metabolic dysregulation, mainly concerning the risk for diabetes. Consequently, undiagnosed and untreated OSA is a significant burden on the healthcare system, with increased healthcare utilization seen in those with untreated OSA, highlighting the importance of early and accurate diagnosis of this common disorder, as just pointed out (24). Therefore, recognizing and adequately treating OSA is a compelling issue for these copious reasons.
The treatment of OSA has been shown to improve quality of life, lower the rates of motor vehicle accidents, and reduce the risk of the chronic health consequences of untreated OSA mentioned above (25). There are also data supporting a decrease in healthcare utilization and cost following the diagnosis and treatment of OSA (26). However, there are challenges and uncertainties in making the management. In this regard, CPAP has low level of acceptance, compliance, and adherence; consequently, new strategies are attempted.
Nas-air® is a new internal nasal dilator that has been found able to significantly reduce snoring (15). The current study demonstrated that this device was able to significantly reduce two relevant respiratory outcomes, such as the apnea-hypopnea index and the oxygen desaturation index, notably there was also a positive trend for the reduction of total sleep time with HbO2 <90%). Nas-air® was also able to significantly improve restorative sleep performance.
These outcomes are consistent with previous studies exploring the capability of nasal dilators to improve sleep-related disorders (11, 13). However, the present preliminary experience was conflicting with other studies (10, 14).
On the other hand, our study has some limitations, including the open study design, the lack of follow-up, and the low number of enrolled patients. Thus, further studies should be conducted to answer these unmet needs. Another interesting future extension of this study could be the use in patients with mild OSA to test the hypothesis that the use of an internal nasal dilator may avoid CPAP therapy. Anyway, the strength of the current study was the demonstration that a single application of the device was able to significantly improve respiratory outcomes and consequently improve the quality of the sleep.
In conclusion, this study showed that Nas-air® is a new internal nasal dilator potentially capable to significantly improve respiratory outcomes and sleep quality.
Conflict of interest:
None to declare
References
- 1.Akinussi M, Saliba R. Emerging therapies for obstructive sleep apnea. Lung. 2012;190:365–71. doi: 10.1007/s00408-012-9380-1. [DOI] [PubMed] [Google Scholar]
- 2.Freedman N. Improvements in current treatments and emerging therapies for adult obstructive sleep apnea. F1000Prime Reports. 2014;6:36. doi: 10.12703/P6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim J, In K, You S, Kang K, Shim J, Lee S, Lee J, Park C, Shin C. Prevalence of sleep-disordered breathing in middle-aged Korean men and women. Am J Respir Crit Care Med. 2004;170:1108–13. doi: 10.1164/rccm.200404-519OC. [DOI] [PubMed] [Google Scholar]
- 4.Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373:82–93. doi: 10.1016/S0140-6736(08)61622-0. [DOI] [PubMed] [Google Scholar]
- 5.Johnson KG, Johnson DC. Frequency of sleep apnea in stroke and TIA patients: a meta-analysis. J Clin Sleep Med. 2010;6:131–7. [PMC free article] [PubMed] [Google Scholar]
- 6.Sanders MH, Montserrat JM, Farre R, Givelber RJ. Positive pressure therapy: a perspective on evidence-based outcomes and methods of application. Proc Am Thorac Soc. 2008;5:161–72. doi: 10.1513/pats.200709-150MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jordan AS, McSharry DG. Malhotra A: Adult obstructive sleep apnoea. Lancet. 2014;383:736–47. doi: 10.1016/S0140-6736(13)60734-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varendh M, Andersson M, Bjornsdottir E, Hrbos-Strom H, Johannisson A, Arnardottir ES, et al. Nocturnal nasal obstruction is frequent and reduces sleep quality in patients with obstructive sleep apnea. J Sleep Res. 2018;27(4):e12631. doi: 10.1111/jsr.12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nigro CEN, Nigro JFA, Mello JF. Nasal valve: anatomy and physiology. Braz J Otorhinolaryngol. 2009;75:305–10. doi: 10.1016/S1808-8694(15)30795-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leitzen KP, Brietzke SE, Lindsay RW. Correlation between nasal anatomy and objective obstructive sleep apnea severity. Otolaryngol HNS. 2014;150:325–31. doi: 10.1177/0194599813515838. [DOI] [PubMed] [Google Scholar]
- 11.Colrain IM, Brooks S, Black J. A pilot evaluation of a nasal expiratory resistance device for the treatment of obstructive sleep apnea. J Clin Sleep Med. 2008;4:426–33. [PMC free article] [PubMed] [Google Scholar]
- 12.Schonhofer B, Franklin KA, Brunig H, Wehde H, Kohler D. Effect of nasal-valve dilation on obstructive sleep apnea. Chest. 2000;118:587–90. doi: 10.1378/chest.118.3.587. [DOI] [PubMed] [Google Scholar]
- 13.McLean HA, Urton AM, Driver HS, Tan AKW, Day AG, Munt PW, et al. Effect of treating severe nasal obstruction on the severity of obstructive sleep apnea. Eur Respir J. 2005;25:521–7. doi: 10.1183/09031936.05.00045004. [DOI] [PubMed] [Google Scholar]
- 14.Liistro G, Rombaux P, Dury M, Pieters T, Aubert G, Rodenstein DO. Effects of Breathe Right on snoring: a polysomnographic study. Resp Med. 1998;92:1076–8. doi: 10.1016/s0954-6111(98)90358-4. [DOI] [PubMed] [Google Scholar]
- 15.Gelardi M, Porro G, Sterlicchio B, Quaranta N, Ciprandi G. Internal nasal dilatator (Nas-air®) in patients with snoring. J Biol Reg. 2018;32:1267–73. [PubMed] [Google Scholar]
- 16.Chung F, Memtsoudis SG, Ramachandran SK, Nagappa M, Opperer M, Cozowicz C, et al. Society of Anesthesia and Sleep Medicine Guidelines on Preoperative Screening and Assessment of Adult Patients With Obstructive Sleep Apnea. Anesth Analg. 2016;123:452–73. doi: 10.1213/ANE.0000000000001416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 18.Shafazand S. Perioperative management of obstructive sleep apnea: ready for prime time? Cleve Clin J Med. 2009;76(4):S98–103. doi: 10.3949/ccjm.76.s4.16. [DOI] [PubMed] [Google Scholar]
- 19.Drake CL, Hays RD, Morlock R, Wang F, Shikiar R, Frank L, et al. Development and evaluation of a measure to assess restorative sleep. J Clin Sleep Med. 2014;10:733–41. doi: 10.5664/jcsm.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mallampati SR, Gatt SP, Gugino LD. A clinical sign to predict difficult tracheal intubation: a prospective study. Can Anaesth Soc J. 1985;32:429–434. doi: 10.1007/BF03011357. [DOI] [PubMed] [Google Scholar]
- 21.Kapur VK, Auckley DH, Chowdhuri S, Kuhlman DC, Mehra R, Ramar K, et al. Clinical practice guidelines for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine Clinical Practice Guidelines. J Clin Sleep Med. 2017;13:479–504. doi: 10.5664/jcsm.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population-a review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7:1311–1322. doi: 10.3978/j.issn.2072-1439.2015.06.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Budhiraja R, Budhiraja P, Quan SF. Sleep-disordered breathing and cardiovascular disorders. Respir Care. 2010;55:1322–32. [PMC free article] [PubMed] [Google Scholar]
- 24.Kapur V, Blough DK, Sandblom RE, et al. The medical cost of undiagnosed sleep apnea. Sleep. 1999;22:749–55. doi: 10.1093/sleep/22.6.749. [DOI] [PubMed] [Google Scholar]
- 25.Kakkar RK, Berry RB. Positive airway pressure treatment for obstructive sleep apnea. Chest. 2007;132:1057–72. doi: 10.1378/chest.06-2432. [DOI] [PubMed] [Google Scholar]
- 26.Kapur VK. Obstructive sleep apnea: diagnosis, epidemiology, and economics. Respir Care. 2010;55:1155–67. [PubMed] [Google Scholar]