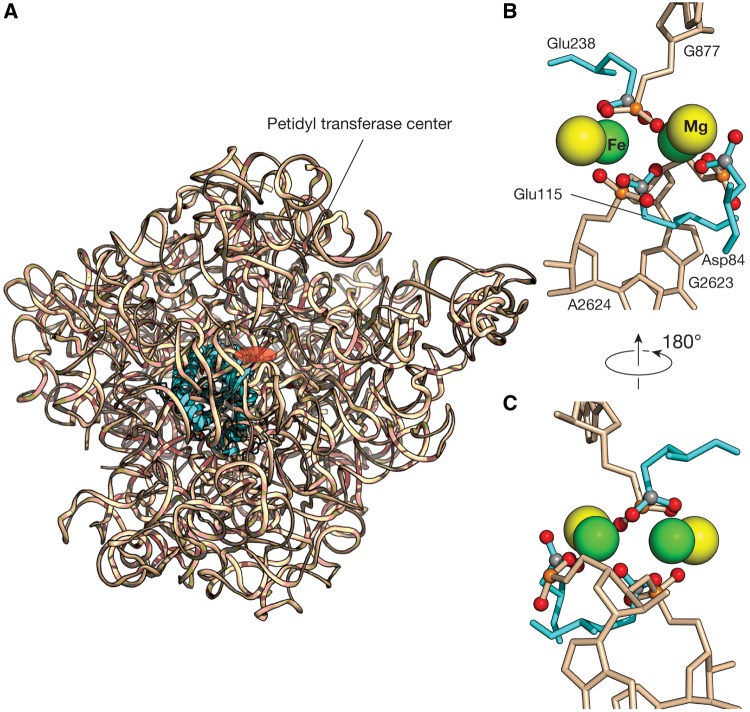
Fig. 2.
The superimposition of the Mg2+-μc and the dinuclear iron clusters. (A) One of the Mg2+-μc’s (Hsiao and Williams 2009) that is closest to the PTC (red circle) of the Haloarcula marismortui 23S rRNA (wheat ribbon, PDB entry 1JJ2) was superimposed to the catalytic center of the Escherichia coli RNR R2 (cyan ribbon, PDB entry 1PIY). (B) A close-up view of the catalytic site of this superimposition shows the di-Fe2+ geometry in RNR R2 is well conserved in Mg2+-μc of the 23S rRNA. The three carboxylic groups of the amino acid residues that are directly contacted by the Fe2+ are mimicked by the phosphate groups of the nucleotides that are direct contact with the Mg2+. (C) Another view of this superimposition. Green spheres denote Fe2+, yellow: Mg2+, orange: phosphorous, red: oxygen, and gray: carbon. RNA is colored wheat, and protein colored cyan.