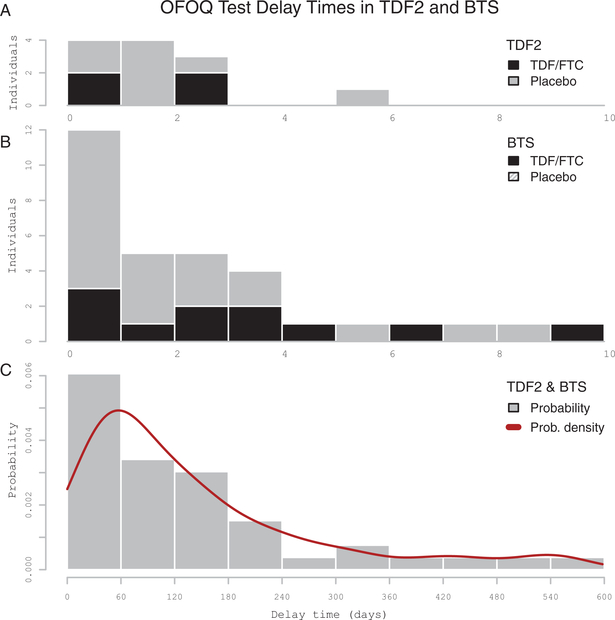
Figure 1.
Distribution of estimated delay times between human immunodeficiency virus type 1 infection and oral fluid OraQuick (OFOQ) test positivity. A and B, Delay times for the Botswana TDF/FTC Oral HIV Prophylaxis Trial (TDF2) and Bangkok Tenofovir Study (BTS) participants, respectively. Participants received either tenofovir disoproxil fumarate (TDF) or TDF/emtricitabine (FTC)-based preexposure prophylaxis (black columns) or placebo (gray columns). C, Delay time probability distribution for TDF2 and BTS. Estimated delay time calculated using the midpoint method. Data are only shown for participants with delay >0 days. The x-axis shows delay time (days); the y-axis shows counts of individuals with false-negative tests by estimated delay time (A and B) and probability density (C).