Abstract
Current standard of care for patients with Hodgkin’s and non-Hodgkin’s lymphoma is high does conditioning with autologous stem cell transplantation (ASCT). For some patients (i.e., highest risk disease, insufficient stem cell number after mobilization, or patients whose bone marrow is involved with the disease) allogeneic transplantation (alloHCT) offers potential cure. However, majority of patients undergoing alloHCT, receive reduced intensity conditioning as preparative regimen and studies assessing outcomes of patients after alloHCT with myeloablative conditioning (MAC) are limited. In this retrospective study, we reviewed outcomes of 22 patients with recurrent and refractory lymphoma who underwent alloHCT with myeloablative BEAM conditioning and received tacrolimus/sirolimus as GVHD prophylaxis at City of Hope from 2005 to 2018. With a median follow-up of 2.6 years (range: 1.0-11.2), probability of 2 year overall survival and event-free survival were 58.3% (95% CI: 35.0 – 75.8) and 45.5% (95% CI: 24.4 – 64.3), respectively. Cumulative incidence of acute graft-versus-host disease (GVHD) grade II-IV was 45.5 (95% CI: 23.8 – 64.9) with only one patient developing grade IV acute GvHD. However, chronic GVHD was seen in 55% of patients (n=12). Of the 22 eligible patients, 2 had prior ASCT and 2 had prior alloHCT. Both patients with prior ASCT developed severe regimen-related toxicity. Patients who underwent alloHCT with chemorefractory disease had lower survival rate with 1-year OS and EFS of 44.4% and 33.0%, respectively. In conclusion, alloHCT with BEAM preparative regimen and Tac/Siro-based GVHD should be considered as an alternative option for patients with highest-risk lymphoma whose outcomes are expectedly poor after ASCT.
INTRODUCTION
Autologous stem cell transplantation (ASCT) with high dose conditioning is the current standard of care for patients with relapsed non-Hodgkin’s lymphoma (NHL) or Hodgkin’s lymphoma (HL).1,2 Outcomes of a large retrospective study done by the Center for Blood and Marrow Transplant Research (CIBMTR), comparing the impact of several commonly used high-dose therapy regimens in patients with NHL and HL demonstrated that among patients with HL, BEAM (BCNU, etoposide, cytarabine and melphalan) regimen was associated with better survival compared to all other regimens, and indicated that there is variability in toxicity and disease outcomes among specific ASCT regimens.3 In a more recent retrospective multicenter study, Herrera et al, reported that patients with relapsed/refractory NHL, double-hit lymphoma (chromosomal rearrangements in BCL2 and MYC) or double-expressor lymphoma (coexpression of BCL2 and MYC by IHC) tend to have inferior outcomes post ASCT, compared to relapsed/refractory patients lacking these high risk features.4 The low survival rates in these high-risk patients illustrate the need for novel/investigational therapies beyond high dose chemotherapy and ASCT, including allogeneic hematopoietic cell transplantation (alloHCT).
For NHL/HL patients with highest risk disease, patients without sufficient number of stem cells due to inefficient mobilization, or patients whose bone marrow is involved with lymphoma, alloHCT offers a potential cure. Use of alloHCT with reduced intensity conditioning (RIC) for relapsed and refractory diffuse large B cell lymphoma (DLBCL) patients with prior unsuccessful ASCT, multiple salvage therapies, advanced age, and/or medical comorbidities has been reported by multiple investigators, with favorable overall survival (OS) of 28-49%.5-8 However, studies assessing outcomes of patients after alloHCT with myeloablative conditioning (MAC) are limited. In one retrospective study, alloHCT with MAC was used for NHL with 2 year OS of 45%, but with high risk of toxicities and treatment related mortality (TRM).9 Due to the relatively limited use of (MAC) in alloHCT, no standard MAC regimen has been established for patients with NHL/HL. Table 1 is the summary of recent studies reporting alloHCT outcomes in patient with recurrent and refractory lymphomas.
Table 1.
Summary of previous reports of allogeneic transplant outcomes in patients with recurrent/refractory lymphoma
| N of patients |
Age (Range) |
Resistant disease (N) | Donor Type | Conditioning Regimen |
NRM (years) |
Relapse (years) |
OS (Years) |
PFS (years) |
aGVHD | cGVHD | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Truelove et al.12 | 46 | 44.8 (18-59) | PR: (34); CR-1: (22) |
MSD: 69 MUD: 23% mMUD: 6.6% |
BEAM CAMPATH | 11%(5) | 53%(5) | 42%(5) | 36%(5) | 15% | 28% |
| Law et al.37 | 31 | 36 (20-60) | CR: (8); PR: (15); IF: (8) |
MSD | CBV | 31%(1) | 25%(1) | 47%(1) | 44%(1) | 29% | 39% |
| Rossi et al.38 | 18 | 42 (18-55) | CR: (10); Resistant: (5); Therapy related death: (3) |
MSD | CBV | 17%(3) | 27%(3) | 76%(2) | 56%(2 ) | 22% | 22% |
| Lazrus 39 | 79 | 46 (21-59) | 42% | MRD: 100% | CY+TBI (12Gy)/+ BU (82%) | 28%(3) | 33%(5) | 52%(3) | 22%(5) | 42% | 26% |
| Van Kampen et al.40 | 101 | 46 (18-66) | 26% | MRD: 71% MUD: 29% |
CY+TBI (12Gy)/± VP-16 + BU (82%) | 28%(3) | 30%(3) | 52%(3) | 42%(3) | 51% | 42% |
| Bacher et al.41 | 396 | 48 (18-69) | 32% | MRD: 33% MUD: 67% |
MAC: CY+TBI (12GY)/+ BU (77%) | 56%(5) | 26%(5) | 18%(5) | 18%(5) | 43% | 37% |
| Rezvani et al.42 | 32 | 52 (18-67) | 72% | MRD: 66% MUD: 34% |
Flu/TBI (2Gy) | 25%(3) | 41%(3) | 45%(3) | 35%(3) | 53% | 44% |
| Hamdani et al.8 | MAC: 307 | 46 (19-66) | 100% | MSD: 60% MUD: 30% mMUD: 10% |
Cy/TBI; BuCy | 53%(3) | 28%(3) | 19%(3) | 19%(3) | 29% | 33% |
| RIC: 226 | 53 (20-70) | 100% | MSD: 47% MUD: 31% MSD: 47% |
Flu/Mel; Flu/Bu ± TBI |
42%3) | 35%(3) | 28%(3) | 23%(3) | 31% | 38% | |
| Glass et al.43 | 84 | 48 (18-65) | Rituxan: 55% | MSD: 31% MUD: 33% mMUD: 36% |
Flu/Bu/CY+ Rituxan | 34%(1) | 29%(1) | 52%(4) | 45%(1) | 46% | 33% |
| No rituxan: 55% | MSD: 24% MUD: 48% mMUD: 26% |
Flu/Bu/CY | 37%(1) | 42% | 41% | ||||||
| Thomson et al.7 | 48 | 46 (23-64) | 17 | MRD: 62% MUD: 38% |
Flu/Mel + CAMPATH | 32%(4) | 33%(4) | 47%(4) | 48%(4) | 17% | 22% |
| Brammer et al.31 | 22 | 46 (20-62) | 9% | Haplo | Flu/Mel/TBI (2Gy) | 20%(1) | 27%(2) | 54%(2) | 54%(2) | 51% | 3% |
| Deithrich et al. | 59 | N/A | N/A | Haplo | Flu/Bu ± TBI or Flu/TBI | 25%(2) | 27%(2) | 56%(2) | 50%(2) | ||
| Kanate et al.32 | 185 | 55 (18-75) | 5.4 | Haplo | Flu/Cy/TBI (2gy) | 16%(2) | 34%(2) | 63%(2) | 50%(2) | 8% | 13% |
Feasibility and tolerance of BEAM as a preparative regimen for alloHCT with tacrolimus and methotrexate (Tac/MTX) as graft-versus-host disease (GVHD) prophylaxis in patients with primary refractory or recurrent low grade lymphomas was reported for the first time in 1999 by Przepiorka et al,.10 Results of Przepiorka’s study were later confirmed by other groups administering BEAM with Campath as GVHD prophylaxis.11,12
Aberrant cell signaling through the mammalian target of rapamycin (mTOR), phosphatidylinositol-3-kinase/Akt (PI3K/Akt) pathways is shown to be associated with increased metastatic potential and cell proliferation and resistance to chemotherapy in both NHL and HL. mTOR inhibitors (i.e., sirolimus) have demonstrated promising results in treatment of lymphoid malignancies.13-15 We and others have evaluated a combination of tacrolimus/sirolimus (Tac/Sir) as GVHD prophylactic regimen after alloHCT, and demonstrated that this combination is associated with reduced incidence/severity of acute GVHD and NRM.16-22 In lymphoma patients, administration sirolimus has been shown to be associated with improved OS after alloHCT in the RIC setting.23 Results of this retrospective study led to a randomized trial comparing the outcomes between Tac/MTX and Tac/Sir/MTX in RIC HCT. In this trial, addition of sirolimus for GVHD prophylaxis was found to be associated with no increased overall toxicity and a lower risk of acute GVHD, albeit without improving patients’ survival.24
Based on this background, we performed a retrospective study, aiming to assess the efficacy of combining alloHCT with BEAM preparative regimen and Tac/Siro-based GVHD prophylaxis in patients with recurrent and refractory lymphoma.
METHODS
Study Population
IRB approval was obtained to review medical records of lymphoma patients who underwent alloHCT and were conditioned with BEAM from 2005 to 2018 at City of Hope. A total of 28 patients were identified. Patients who received transplant from a syngeneic donor (n=5) or received Tac/MTX as GVHD prophylaxis (n=1) were excluded. The remaining 22 patients were included for the final analysis. Majority of patients were selected for alloHCT due to the high risk of relapse and best clinical judgment by the treating physician. The disease risk index (DRI) assignment tool 25 was used to retrospectively analyze predicted survival outcome post alloHCT.
Transplant procedure:
All patients received high dose conditioning with BEAM regimen as follows: BCNU 300 mg/m2 on day −6; etoposide 200 mg/m2 on days −5 to −2 (total dose 800 mg/m2) cytarabine 400 mg/m2 on days −5 to −2 (total dose of 1600 mg/m2) and melphalan 140 mg/m2 on day −1. Allogeneic stem cells were infused on day 0. GVHD prophylaxis comprised of intravenous (IV) tacrolimus infusion starting at 0.02mg/kg started on day −3 and sirolimus 12 mg loading dose on day-3 followed by 4 mg oral daily. Sirolimus and tacrolimus levels were subsequently adjusted based on trough levels checked twice weekly. Patients were monitored for lab features of thrombotic microangiopathy. All patients received standard antimicrobial prophylaxis with Bactrim loading till day −3, micafungin 50 mg (IV) daily starting day +1 and acyclovir for zoster prophylaxis on day −1.
Outcome Definitions and Statistical Analyses
Descriptive statistics were used to summarize baseline patient demographic, treatment, and disease characteristics. Survival estimates were calculated using the Kaplan-Meier method. OS was defined as time from transplant to death of any kind, while event free survival (EFS) was defined as time from transplant to relapse/progression or death of any kind. Cumulative incidence of relapse/progression, non-relapse mortality (NRM), acute GVHD and chronic GVHD were estimated using competing risks. Relapse/progression was defined as time from transplant to relapse or progression with NRM as a competing risk. NRM was defined as time from transplant to death from any cause other than relapse/progression with relapse or progression as a competing risk. Acute GVHD was defined as time to grade II-IV aGVHD onset with relapse and death as competing events. Chronic GVHD was defined as time to any cGVHD onset with relapse and death as competing events.
RESULTS
Patient characteristics
Median age of patients and donors at the time of transplant were 46 (range: 18-61) and 52 years (range: 29-63), respectively. Of the 22 eligible patients, 16 (72.7%) had NHL, 5 had HL (22.7%), and 1 (4%) had histiocytic sarcoma evolved from prior follicular lymphoma. In the NHL subgroup, 10 patients (62.5%) had DLBCL. Majority of patients were selected for alloHCT due to high risk of relapse and 45% of patients (n=10) had primary refractory disease requiring more than one line of salvage chemotherapy to enter remission. Disease status at the time of HCT was i) Complete Remission (CR)-1 in one patient (n=4%) and CR-2 or greater in 9 patients (40.9%); ii) Refractory disease in 9 patients (40.9%) and iii) Partial Response (chemosensitive) in 3 (13.6%) patients (Table 2). Patients were heavily pretreated with a mean of 3.6 (range: 2-7) lines of prior therapy. The DRI score was high risk in n=8 patients and very high risk in n=13 predicting 2 year overall survival of 34% (95% CI 17-31%) respectively –one patient had intermediate risk score. Donors were matched siblings in 11 patients (50.0%) or matched unrelated donors (MUDs). Of the 11 MUD HCT recipients, four (18.2%) received HCT from 9/10 matched donors and their GVHD prophylaxis consisted of Tac/Sir and mini MTX. The remainder of MUD recipients with 10/10 HLA matched donors received Tac/Sir only as GVHD prophylaxis.
Table 2.
Patient and Disease Characteristics
| characteristics | Frequency (percent) Median/Mean (range) |
|---|---|
| Median Age | |
| Patient | 46 (18-61) |
| Donor | 52 (29-63) |
| Gender | |
| Male | 16 (72.7) |
| Female | 6 (27.3) |
| Donor | |
| Male | 14 (63.6) |
| Female | 8 (36.4) |
| Diagnosis | |
| Hodgkin’s Lymphoma | 5 (22.7) |
| NHL | 16 (72.7) |
| DLBCL | 10 (45.5) |
| PTCL | 1 (4.5) |
| BL | 1 (4.5) |
| FL | 1 (4.5) |
| Transformed FL | 1 (4.5) |
| NK/T cell | 1 (4.5) |
| ALCL | 1 (4.5) |
| Histiocytic Sarcoma | 1 (4.5) |
| Disease status | |
| CR-2 or more | 9 (40.9) |
| CR-1 | 1 (4.5) |
| Refractory | 9 (40.9) |
| Partial response | 3 (13.6) |
| Donor type | |
| Sibling | 11 (50.0) |
| Unrelated | 11 (50.0) |
| Graft source | |
| BM | 1 (4.5) |
| PBSCs | 21 (95.5) |
| HLA match | |
| Matched | 18 (81.8) |
| Mismatched | 4 (18.2) |
| GVHD prophylaxis | |
| Tac/siro | 18 (81.8) |
| Tac/siro/MTX | 4 (18.2) |
| Prior HCT | |
| Allogeneic | 2 (9.1) |
| Autologous | 2 (9.1) |
| Mean lines of prior therapy | 3.6 (2-7) |
| DRI score | |
| Intermediate | 1 (4.5) |
| High risk | 8 (36.4) |
| Very high risk | 13 (59.1) |
Transplant outcomes
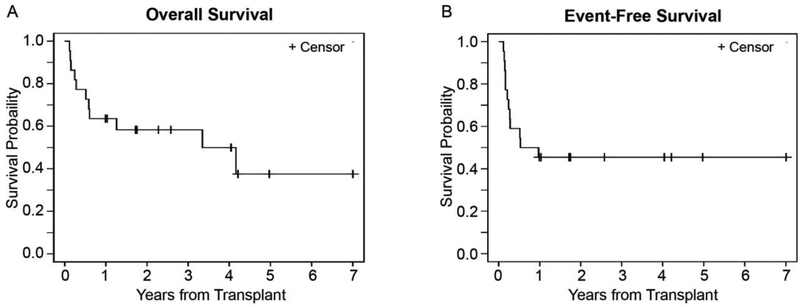
All patients engrafted successfully with a median time to neutrophil engraftment of 13 days (range 10-24 days). The median duration of follow up for living patients was 2.6 years (range: 1.0-11.2), with a 2-year probability of OS and EFS of 58.3% (95% CI: 35.0-75.8) and 45.5% (95% CI: 24.4-64.3), respectively. (Table 4 and Fig. 1 A and B) The cumulative incidence of relapse and NRM at 2 years were 31.8% (95% CI: 13.6-51.8) and 22.7% (95% CI: 8.0-42.0), respectively.(Table 4) Acute GVHD was noted in 11 patients (50%), from which 10 patients developed grade II-IV GVHD with the cumulative incidence of 45.5% (95% CI: 23.8-64.9). Only one patient developed Grade IV acute GVHD. The cumulative incidence of chronic GVHD was 45.5% at 1 year (95% CI: 23.4-65.2) with majority of them having extensive/severe GVHD (n=10). (Table 3)
Table 4.
Point Estimates for Outcomes
| 100days | 1 year | 2 years | |
|---|---|---|---|
| OS | 81.8 (58.8-92.8) | 63.6 (40.3-79.9) | 58.3 (35-78.5) |
| EFS | 63.6 (40.3-79.9) | 45.5 (24.4-64.3) | 45.5 (24.4-64.3) |
| NRM | 18.2 (5.5-36.8) | 22.7 (8.0-42.0) | 22.7 (8.0-42.0) |
| CIR | 18.2 (5.4-36.9) | 31.8 (13.6-51.8) | 31.8 (13.6-51.8) |
| aGVHD | 45.5 (23.8-64.9) | ||
| cGVHD | 45.5 (23.4-65.2) |
Figure 1.
Survival probability curves. (A) OS. (B) EFS.
Table 3.
Transplant outcomes
| Outcomes (n=22) | Frequency (%) Median (range) |
|---|---|
| Acute GVHD | |
| Yes | 11 (50.0) |
| Grade I | 1 |
| Grade II | 8 |
| Grade III | 1 |
| Grade IV | 1 |
| NO | 11 (50.0) |
| Chronic GVHD | |
| Yes | 12 (54.5) |
| Limited | 2 |
| Extensive | 10 |
| No | 5 (22.7) |
| Not evaluable ( died <100 days) | 5 (22.7) |
| Relapse | |
| Yes | 7 (31.8) |
| No | 15 (68.2) |
| Vital Status | |
| Alive | 11 (50) |
| Dead | 11 (50) |
| Cause of death | |
| Disease progression | 4 (36.4) |
| Infection | 1 (9.1) |
| DAH | 1 (9.1) |
| aGVHD | 1 (9.1) |
| cGVHD | 1 (9.1) |
| ARDS/MODS | 1 (9.1) |
| UK | 2 (18.2) |
Sinusoidal obstruction syndrome (SOS) was noted in 4 patients (18%). All 4 patients were successfully treated with defibrotide with complete resolution of clinical symptoms and radiologic features. Two of these 4 patients who developed SOS had had prior alloHCT. One of the four patients who developed SOS, had multiple rounds of chemotherapy (CR-3) and the fourth patient had intestinal T cell lymphoma with hepatic involvement at diagnosis. Post-transplant thrombotic microangiopathy (TMA) was diagnosed in 2 patients (9%) in our cohort.
Causes of death (n=11) were: disease progression (n=4), infection (n=1), diffuse alveolar hemorrhage (n=1), acute GVHD (n=1), chronic GVHD (n=1), sepsis and multi-organ failure (n=1), and unknown in two cases. (Table 3). Although the incidence of acute and chronic GVHD was high in our cohort, GVHD related mortality rates were low, with only two patients dying from complications due to either acute or chronic GVHD. The incidence of acute and chronic GVHD in this report is consistent with the prevailing rates at our institution.
Impact of prior transplant
Four patients in this study had prior transplant – 2 allogeneic and 2 autologous HCT. Both patients who received ASCT using BEAM conditioning developed severe regimen-related toxicity and passed away from pulmonary complications, presumably related to cumulative pulmonary toxicity from repeat BCNU exposure. One patient with prior alloHCT developed gastrointestinal (GI) GVHD and fungal pneumonia and died. Among 18 patients excluding four with prior HCT, the 2-year OS and EFS were 65.7% (95% CI: 38.7-83.0) and 50.0% (95% CI: 25.9-70.1), respectively.
Adverse outcomes were noted in chemorefractory (n=9) patients when compared to chemosensitive (n=13) patients prior to allogeneic transplant. The 2-year OS and EFS estimates were 33.3% (95% CI: 7.8-62.3) and 33.3% (95% CI: 7.8-62.3), respectively, in chemorefractory patients compared to 76.9% (95% CI 44.2-91.9) and 53.8% (95% CI 24.8-76.0) in patients with chemosensitive lymphoma pre alloHCT. No difference was noted for any of the clinical endpoints when patients with Hodgkin’s and non-Hodgkin’s lymphoma were compared.
DISCUSSION
Advances in immuno-chemotherapy in the past decade have helped improving clinical outcomes of patients diagnosed with NHL and HL. The most recent Surveillance, Epidemiology, and End Results (SEER) database from 2008-2014 shows a 5-year survival of 71.4% and 86.6% for patients diagnosed with NHL and HL, respectively. Unfortunately, relapse and refractory disease remains a significant challenge in treatment of these patients with a uniformly poor prognosis.26-28 In a recent retrospective study, patients with aggressive chemo-resistant B cell lymphomas relapsed within one year post-ASCT with a reported median OS of six months.29 In a Collaborative Trial in Relapsed Aggressive Lymphoma (CORAL) study, subgroup of patients failing rituximab-based treatments within 12 months of diagnosis had poor outcomes with 3 year PFS of only 23% with ASCT,30 demonstrating a major limitation in treating high risk patients with ASCT and the need for developing more effective therapies including alloHCT. However, the most effective conditioning regimen for alloHCT in the setting of aggressive lymphomas remains unknown.
Feasibility and tolerance of BEAM as a preparative regimen for alloHCT and Tac/MTX-based GVHD prophylaxis, was first described by Przepiorka et al,10 in 30 patients with refractory or recurrent low and intermediate grade lymphoma. Twenty-three patients achieved a complete remission post allograft and two-year relapse rate was 23%, survival was 48%, and disease-free survival (DFS) was 42%. In aggressive lymphoma setting, Truelove et al,12 reported outcomes of 46 patients with relapsed refractory aggressive NHL who underwent alloHCT with BEAMCAMPATH preparative regimen, with OS of 54% and 42% and PFS of 41% and 36% at 1 and 5 years, respectively.
Recently, promising results have been reported in haploidentical transplant setting using RIC with 2 year PFS and relapse rates of 54% and 27%, respectively.31 Similar results were reported by Kanate et al, in RIC setting with 3 year PFS and relapse rates of 47% and 36% with very low rates of acute and chronic GVHD (8% and 13%, respectively).32 However, in both studies, the number of patients with chemorefractory disease in haploidentical RIC arm were low (9% and 5%, respectively) and prospective studies for patients with chemo-resistant disease are desirable, to see if PFS benefit is seen in these high risk patients. Table 1 is a summary of previous reports of transplant outcomes in patients with refractory and recurrent leukemia.
Using BEAM conditioning with Tac/Siro as GVHD prophylaxis, our outcomes (2-year OS: 65.7%, EFS: 50% in 18 patients without prior transplant) compares favorably to previous reports.12 It is important to note that, while the number of patients included in this study were small, our patient population had higher risk of disease relapse based on disease status at the time of alloHCT compared to other reports. Improved EFS could be due to a combination of BEAM conditioning and anti -lymphoma activity conferred by sirolimus used as part of GVHD prophylaxis. In our cohort, rates of acute and chronic GVHD were high (50%) for these high risk patients, indicating that better GVHD prophylaxis regimens are needed to improve GFRS outcome. Given the high risk of post-HCT relapse, it is possible that treating physicians may have been more aggressive in terms of tapering off immune suppression. Strategies such as post-transplant high-dose cyclophosphamide have the potential to reduce rates of GVHD-related morbidity, although this type of post-transplant treatments should be balanced with relapse risk.
In this high-risk population with aggressive lymphoma undergoing BEAM conditioning and Tac/Siro based prophylaxis two subgroups had worse outcomes. First group was patients who underwent alloHCT with chemorefractory disease, associated with 1-year OS and EFS of 44.4% and 33.0%, respectively- indicating the need to optimize disease control prior to alloHCT. Effective graft-versus-lymphoma activity develops over a period of 3-6 months post alloHCT and our results indicates that increasing conditioning intensity by using BEAM is not sufficient to overcome the high relapse rates associated with chemorefractory lymphoma. Second subgroup was patients with prior allogeneic or autologous HCT, associated with unacceptably high risk of NRM due to pulmonary toxicity, SOS, and severe GVHD/infection in our 4 patients. Based on our data, caution is indicated prior to recommending the use of BEAM-alloHCT in patients with prior HCT and chemorefractory lymphomas.
Lastly, with the advent of immunotherapy outcomes for high-risk patients with recurrent refractory lymphoma have been revolutionized. Neelapu et al,33 reported results of a phase 2 study in 111 patients with refractory B cell lymphomas who were treated with anti-CD19 CAR T cells (axicabtagene ciloleucel), showing overall response rates of 82% and complete response rates of 54%. The OS of patients at 18 months was 52%, with primary grade 3 toxicity of cytokine-release syndrome and neurologic toxicity of 13% and 28%, respectively. Similarly, exciting outcomes have been reported with checkpoint inhibitors in relapsed refractory HL with overall response rate of 78% with 17% complete responses.34 However, long-term follow-up regarding efficacy of CAR T cell therapy is not available yet, and to date no survival plateau has been achieved for checkpoint inhibition in relapsed and refractory HL.35,36 Therefore, alloHCT may have a continued role for disease control in the subset of NHL and HL34 patients with incomplete remissions post chemotherapy, cellular therapy or checkpoint inhibitor therapy.
In conclusion, our results demonstrates that alloHCT with BEAM preparative regimen and Tac/Siro-based GVHD offers an alternative option for patients with highest-risk lymphoma whose outcomes are expectedly poor after ASCT.
HIGHLIGHTS.
BEAM with Tac/Siro is an alternative therapy for highest-risk lymphoma patients.
Favorable survival outcomes were achieved with 2 year OS of 65.7% and EFS of 50%.
High rates of GVHD indicates the need for better GVHD prophylactic regimen.
Subgroups of patients who underwent previous HCT had worse outcomes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin's lymphoma. N Engl J Med. 1995;333(23):1540–1545. [DOI] [PubMed] [Google Scholar]
- 2.Schmitz N, Pfistner B, Sextro M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: a randomised trial. Lancet. 2002;359(9323):2065–2071. [DOI] [PubMed] [Google Scholar]
- 3.Chen YB, Lane AA, Logan B, et al. Impact of conditioning regimen on outcomes for patients with lymphoma undergoing high-dose therapy with autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2015;21(6):1046–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herrera AF, Mei M, Low L, et al. Relapsed or Refractory Double-Expressor and Double-Hit Lymphomas Have Inferior Progression-Free Survival After Autologous Stem-Cell Transplantation. J Clin Oncol. 2017;35(1):24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sirvent A, Dhedin N, Michallet M, et al. Low nonrelapse mortality and prolonged long-term survival after reduced-intensity allogeneic stem cell transplantation for relapsed or refractory diffuse large B cell lymphoma: report of the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire. Biol Blood Marrow Transplant. 2010;16(1):78–85. [DOI] [PubMed] [Google Scholar]
- 6.Fenske TS, Hamadani M, Cohen JB, et al. Allogeneic Hematopoietic Cell Transplantation as Curative Therapy for Patients with Non-Hodgkin Lymphoma: Increasingly Successful Application to Older Patients. Biol Blood Marrow Transplant. 2016;22(9):1543–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomson KJ, Morris EC, Bloor A, et al. Favorable long-term survival after reduced-intensity allogeneic transplantation for multiple-relapse aggressive non-Hodgkin’s lymphoma. J Clin Oncol. 2009;27(3):426–432. [DOI] [PubMed] [Google Scholar]
- 8.Hamadani M, Saber W, Ahn KW, et al. Impact of pretransplantation conditioning regimens on outcomes of allogeneic transplantation for chemotherapy-unresponsive diffuse large B cell lymphoma and grade III follicular lymphoma. Biol Blood Marrow Transplant. 2013;19(5):746–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim S-W, Tanimoto TE, Hirabayashi N, et al. Myeloablative allogeneic hematopoietic stem cell transplantation for non-Hodgkin lymphoma: a nationwide survey in Japan. Blood. 2006;108(1):382–389. [DOI] [PubMed] [Google Scholar]
- 10.Przepiorka D, van Besien K, Khouri I, et al. Carmustine, etoposide, cytarabine and melphalan as a preparative regimen for allogeneic transplantation for high-risk malignant lymphoma. Ann Oncol. 1999;10(5):527–532. [DOI] [PubMed] [Google Scholar]
- 11.Faulkner RD, Craddock C, Byrne JL, et al. BEAM-alemtuzumab reduced-intensity allogeneic stem cell transplantation for lymphoproliferative diseases: GVHD, toxicity, and survival in 65 patients. Blood. 2004;103(2):428–434. [DOI] [PubMed] [Google Scholar]
- 12.Truelove E, Fox C, Robinson S, et al. Carmustine, etoposide, cytarabine, and melphalan (BEAM)- campath allogeneic stem cell transplantation for aggressive non-hodgkin lymphoma: an analysis of outcomes from the British Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2015;21(3):483–488. [DOI] [PubMed] [Google Scholar]
- 13.Witzig TE, Reeder CB, LaPlant BR, et al. A phase II trial of the oral mTOR inhibitor everolimus in relapsed aggressive lymphoma. Leukemia. 2011;25(2):341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ansell SM, Tang H, Kurtin PJ, et al. Temsirolimus and rituximab in patients with relapsed or refractory mantle cell lymphoma: a phase 2 study. Lancet Oncol. 2011;12(4):361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Renner C, Zinzani PL, Gressin R, et al. A multicenter phase II trial (SAKK 36/06) of single-agent everolimus (RAD001) in patients with relapsed or refractory mantle cell lymphoma. Haematologica. 2012;97(7):1085–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cutler C, Logan B, Nakamura R, et al. Tacrolimus/sirolimus vs tacrolimus/methotrexate as GVHD prophylaxis after matched, related donor allogeneic HCT. Blood. 2014;124(8):1372–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodriguez R, Nakamura R, Palmer JM, et al. A phase II pilot study of tacrolimus/sirolimus GVHD prophylaxis for sibling donor hematopoietic stem cell transplantation using 3 conditioning regimens. Blood. 2010;115(5):1098–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cutler C, Li S, Ho VT, et al. Extended follow-up of methotrexate-free immunosuppression using sirolimus and tacrolimus in related and unrelated donor peripheral blood stem cell transplantation. Blood. 2007;109(7):3108–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antin JH, Kim HT, Cutler C, et al. Sirolimus, tacrolimus, and low-dose methotrexate for graft-versus-host disease prophylaxis in mismatched related donor or unrelated donor transplantation. Blood. 2003;102(5):1601–1605. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura R, Palmer JM, O'Donnell MR, et al. Reduced intensity allogeneic hematopoietic stem cell transplantation for MDS using tacrolimus/sirolimus-based GVHD prophylaxis. Leuk Res. 2012;36(9):1152–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khaled SK, Palmer J, Stiller T, et al. A phase II study of sirolimus, tacrolimus and rabbit anti-thymocyte globulin as GVHD prophylaxis after unrelated-donor PBSC transplant. Bone Marrow Transplant. 2013;48(2):278–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pidala J, Kim J, Jim H, et al. A randomized phase II study to evaluate tacrolimus in combination with sirolimus or methotrexate after allogeneic hematopoietic cell transplantation. Haematologica. 2012;97(12):1882–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Armand P, Gannamaneni S, Kim HT, et al. Improved survival in lymphoma patients receiving sirolimus for graft-versus-host disease prophylaxis after allogeneic hematopoietic stem-cell transplantation with reduced-intensity conditioning. J Clin Oncol. 2008;26(35):5767–5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Armand P, Kim HT, Sainvil MM, et al. The addition of sirolimus to the graft-versus-host disease prophylaxis regimen in reduced intensity allogeneic stem cell transplantation for lymphoma: a multicentre randomized trial. Br J Haematol. 2016;173(1):96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Armand P, Kim HT, Logan BR, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123(23):3664–3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hagemeister FB. Treatment of relapsed aggressive lymphomas: regimens with and without high-dose therapy and stem cell rescue. Cancer Chemother Pharmacol. 2002;49 Suppl 1:S13–20. [DOI] [PubMed] [Google Scholar]
- 27.Ardeshna KM, Kakouros N, Qian W, et al. Conventional second-line salvage chemotherapy regimens are not warranted in patients with malignant lymphomas who have progressive disease after first-line salvage therapy regimens. Br J Haematol. 2005;130(3):363–372. [DOI] [PubMed] [Google Scholar]
- 28.Hitz F, Connors JM, Gascoyne RD, et al. Outcome of patients with primary refractory diffuse large B cell lymphoma after R-CHOP treatment. Ann Hematol. 2015;94(11):1839–1843. [DOI] [PubMed] [Google Scholar]
- 29.Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130(16):1800–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28(27):4184–4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brammer JE, Khouri I, Gaballa S, et al. Outcomes of Haploidentical Stem Cell Transplantation for Lymphoma with Melphalan-Based Conditioning. Biol Blood Marrow Transplant. 2016;22(3):493–498. [DOI] [PubMed] [Google Scholar]
- 32.Kanate AS, Mussetti A, Kharfan-Dabaja MA, et al. Reduced-intensity transplantation for lymphomas using haploidentical related donors vs HLA-matched unrelated donors. Blood. 2016;127(7):938–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med. 2017;377(26):2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med. 2015;372(4):311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Armand P, Engert A, Younes A, et al. Nivolumab for Relapsed/Refractory Classic Hodgkin Lymphoma After Failure of Autologous Hematopoietic Cell Transplantation: Extended Follow-Up of the Multicohort Single-Arm Phase II CheckMate 205 Trial. J Clin Oncol. 2018;36(14):1428–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen R, Zinzani PL, Fanale MA, et al. Phase II Study of the Efficacy and Safety of Pembrolizumab for Relapsed/Refractory Classic Hodgkin Lymphoma. Journal of Clinical Oncology. 2017;35(19):2125–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Law LY, Horning SJ, Wong RM, et al. High-dose carmustine, etoposide, and cyclophosphamide followed by allogeneic hematopoietic cell transplantation for non-Hodgkin lymphoma. Biol Blood Marrow Transplant. 2006;12(7):703–711. [DOI] [PubMed] [Google Scholar]
- 38.Rossi HA, Becker PS, Emmons RV, et al. High-dose cyclophosphamide, BCNU, and VP-16 (CBV) conditioning before allogeneic stem cell transplantation for patients with non-Hodgkin's lymphoma. Bone Marrow Transplant. 2003;31(6):441–446. [DOI] [PubMed] [Google Scholar]
- 39.Lazarus HM, Zhang MJ, Carreras J, et al. A comparison of HLA-identical sibling allogeneic versus autologous transplantation for diffuse large B cell lymphoma: a report from the CIBMTR. Biol Blood Marrow Transplant. 2010;16(1):35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Kampen RJ, Canals C, Schouten HC, et al. Allogeneic stem-cell transplantation as salvage therapy for patients with diffuse large B-cell non-Hodgkin's lymphoma relapsing after an autologous stem-cell transplantation: an analysis of the European Group for Blood and Marrow Transplantation Registry. J Clin Oncol. 2011;29(10):1342–1348. [DOI] [PubMed] [Google Scholar]
- 41.Bacher U, Klyuchnikov E, Le-Rademacher J, et al. Conditioning regimens for allotransplants for diffuse large B-cell lymphoma: myeloablative or reduced intensity? Blood. 2012;120(20):4256–4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rezvani AR, Norasetthada L, Gooley T, et al. Non-myeloablative allogeneic haematopoietic cell transplantation for relapsed diffuse large B-cell lymphoma: a multicentre experience. Br J Haematol. 2008;143(3):395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glass B, Hasenkamp J, Wulf G, et al. Rituximab after lymphoma-directed conditioning and allogeneic stem-cell transplantation for relapsed and refractory aggressive non-Hodgkin lymphoma (DSHNHL R3): an open-label, randomised, phase 2 trial. Lancet Oncol. 2014;15(7):757–766. [DOI] [PubMed] [Google Scholar]