Fig. 4|. Equations used to obtain the CSI.
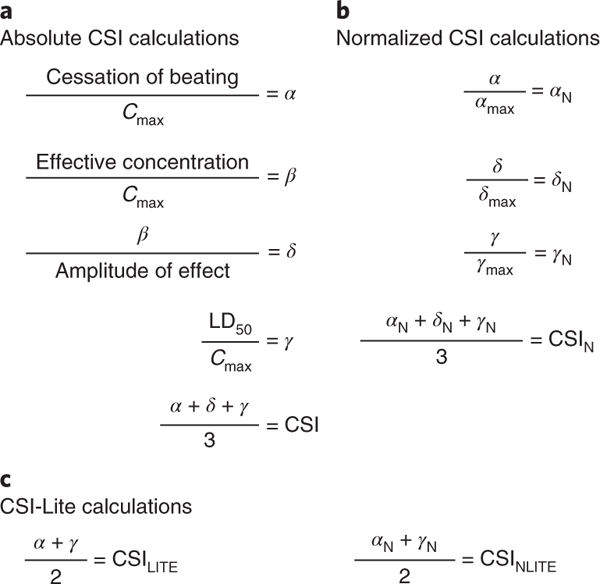
Assume that each metric corresponds to the values associated with a single drug being tested for cytotoxicity and contractility alterations on hiPSC-CMs. a, Metrics α (corrected cessation of beating), β (corrected effective concentration), δ (updated corrected effective concentration), and γ (corrected LD50) are obtained by normalizing values from contractility (cessation of beating, effective concentration, and amplitude of effect) and cytotoxicity (LD50) assays by the maximum blood plasma concentration (Cmax) for each drug being tested on hiPSC-CMs. Cmax values for each drug of interest can be found in FDA clinical trial literature or can be derived from preclinical pharmacokinetic studies in animal models. The absolute α, δ, and γ values are averaged to provide an absolute CSI associated with a specific drug. b, The CSIN metric can be used to evaluate the relative cardiotoxicity of a drug in comparison to other drugs in a large dataset. To derive the CSIN, normalized values for α, δ, and γ (αN, δN, and γN) are obtained by dividing the absoluteα;, δ, and γ values associated with a drug by the maximum α, δ, and γ values in a dataset. CSIN is determined by averaging αN, δN, and γN. c, If it is not possible to obtain δ or δN due to lack of access to a high-throughput contractility analysis platform such as the KIC, a simplified, ‘lite’ version of the final CSI can be obtained by averaging α and γ (CSILITE) or αN and γN (CSINLITE).
