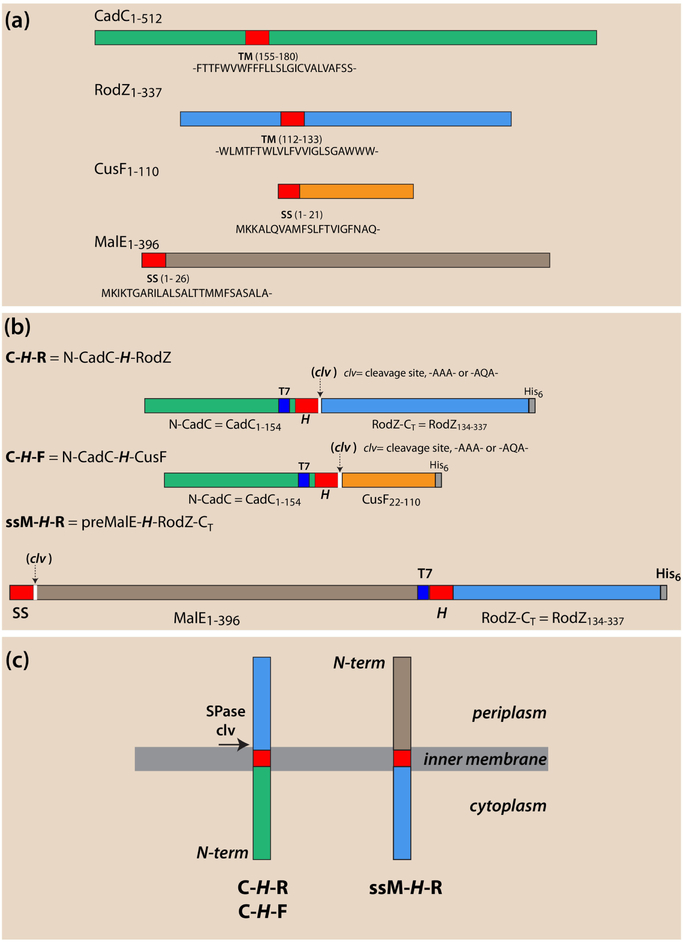
Figure 1.
Schematic overview of single-span proteins and chimeras used in this study. Locations of transmembrane (TM) segments [H-segments] or signal sequences (ss) are colored red. Location of T7 immuno tags are colored blue, His6 tags gray, and cleavage sites white. CadC and RodZ non-TM domains are colored green and blue, respectively, CusF orange, and MalE brown. (a) CadC or RodZ are unusual Type-II (Nin-Cout) single-span membrane proteins (MPs) that lack an N-terminal signal sequence (ss) and have a far-downstream TM segment. CadC activates the CadBA operon during low-pH stress[3–5] while RodZ, plays an important role in the maintenance of the rod shape of Escherichia coli [6, 7]. CusF, is the periplasmic copper chaperone of the E. coli CusCFBA copper-transporting efflux system[14] and MalE is the well-known maltose binding protein [17]. Both have N-terminal signal sequences (ss) indicated in red. (b) Chimeric proteins composed of foldable fragments of the proteins in panel a. Spase I cleavage sites (clv) are inserted immediately following the H-segment except for the ssM-H-R construct that carries a native cleavable signal sequence. Proteins cleaved by SPase I indicate that the protein has entered the SecA pathway and passed through the SecYEG translocon. (c) Schematic overview of the topology of the proteins in panel b after SecA targeting and SecYEG membrane incorporation.