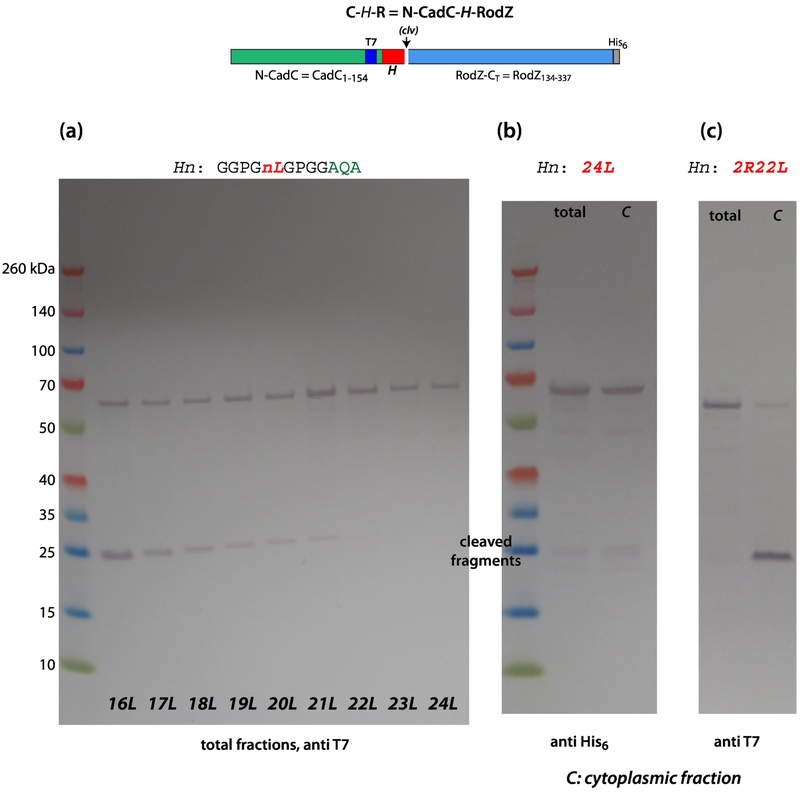
Figure 6.
SecA cannot identify long and highly hydrophobic H-segments. (a) These data show that SecA identification of polyleucine H-segments composed of more than 16 leucines becomes more and more difficult with increasing numbers of leucines, as indicated by the steady decline in the intensity of the cleaved fragments. For segments containing 22 or more residues, the C-H-R constructs were not processed at all. (b) Remarkably, despite the high hydrophobicity of the polyleucine H-segments, the chimeric proteins are found only in the cytoplasmic fraction rather than in inclusion bodies. This might be due to interactions with cytoplasmic chaperones. (c) These data show that replacement of two leucines in the 24Leu construct with two arginine (pos. 10 and 15) transform it to a SecA target (left lane without a cleavage site, right lane with a cleavage site). The occurrence of the cleaved fragment (right lane) indicates SecA targeting. These experiments were carried out using BL21 cells carrying a pET21 vector. Cells were grown in SOC media at 37° C. See Materials and Methods.