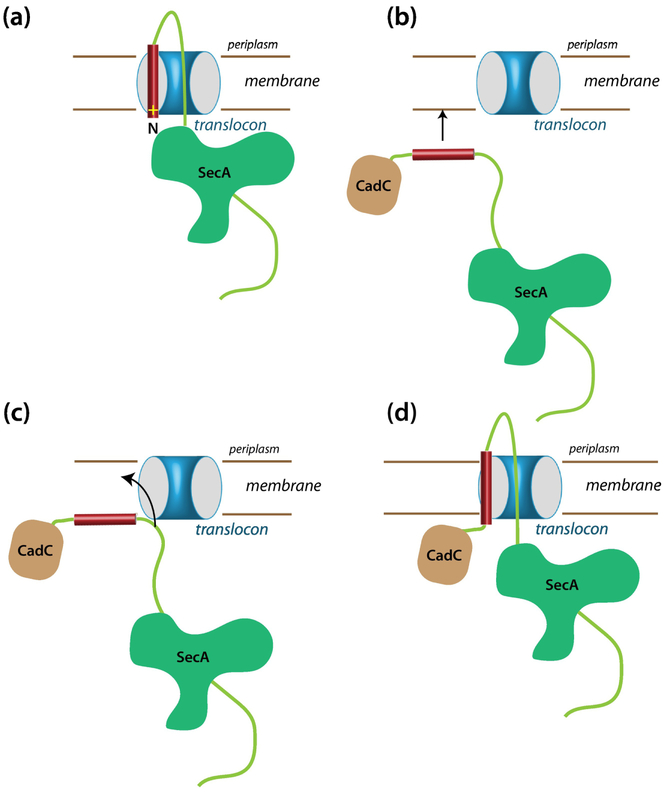
Figure 8.
How can a single-span Type II membrane protein with a far-downstream TM segment be inserted into the membrane via the SecYEG translocon? (a) A typical scheme for the insertion into the translocon of a secreted protein carrying an N-terminal signal sequence. Topologically, this scheme seems unlikely to work for proteins with a far downstream TM domain, because the N-terminal domain is likely already folded when the TM domain emerges from the ribosome. We suggest instead the scheme shown in panels b, c, and d. (b) The folded N-terminal domain problem can be avoided if SecA transports the TM segment to the membrane, bringing it into close proximity of SecYEG. (c) If the affinity of the TM segment for the membrane is higher than for SecA, the segment will bind to the membrane. The nearby translocon would provide a pathway across the membrane for the much more polar part of the chain. For a very hydrophobic segment with a low hydrophobic moment [38, 39], a transmembrane configuration likely has a lower free than a surface-bound state. (d) As the TM segment partitions across the membrane, we suggest that this pulls the more polar C-terminal part of the chain into the translocon so that SecA can secrete the remainder of the chain across the membrane. This scheme works just as well for secreted proteins carrying an N-terminal signal sequence.