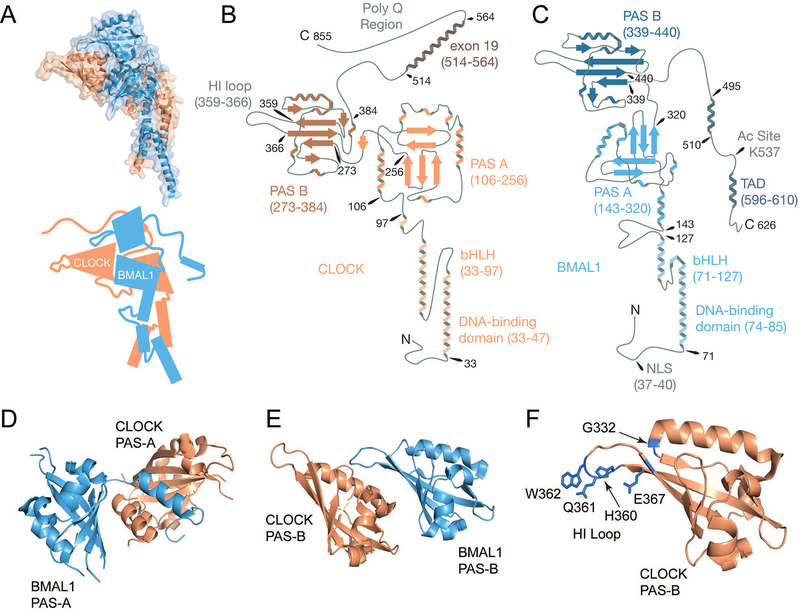
Figure 2. Molecular architecture of the activators CLOCK and BMAL1.
(A) On the top is the crystal structure of the CLOCK/BMAL1 heterodimer, encompassing the bHLH and PAS domains of each protein (PDB: 4F3L). CLOCK is shown in peach and BMAL1 is shown in blue. Below is a cartoon rendering of the heterodimer based on the structure, but with additional disordered regions drawn in. (B) Using available structural data, the structure of CLOCK is rendered in graphical form with domains of interest labeled and numbered based on the amino acid sequence of CLOCK from Mus musculus. (C) The structure of BMAL1 is rendered in graphical form with domains of interest labeled and numbered based on the amino acid sequence of BMAL1 from Mus musculus. (D) The PAS-A domains of CLOCK and BMAL1 have a reciprocal interaction in which the first α-helix of each PAS-A domain binds the β-sheet interface of its partner. (E) The PAS-B domains of CLOCK and BMAL1 interact through the β-sheet interface of BMAL1 and an α-helix of CLOCK, leaving a significant portion of CLOCK’s PAS-B available for other protein-protein interactions. (F) Residues identified as important for interaction between the CLOCK PAS-B domain, primarily its HI loop, and CRY are highlighted on the PAS-B structure in blue.