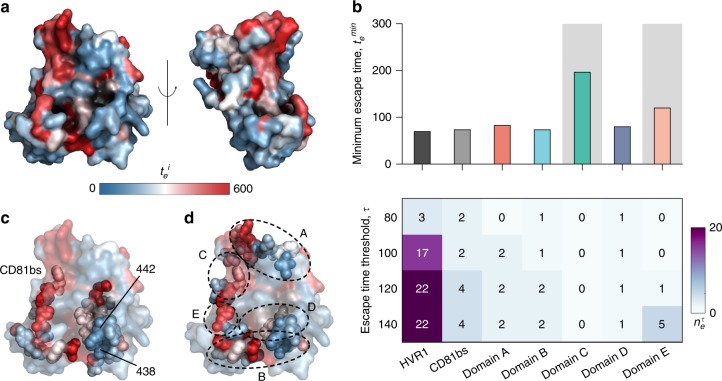
Fig. 3.
Superimposing relative escape times on the available E2 crystal structure reveals difficult to escape regions for antibody targeting. a Residues incurring high escape times upon mutation (large values of ) are shown in red and those associated with low escape times (small values of ) are shown in blue. The crystal structure43 was obtained from the protein data bank with PDB ID 4MWF. Left and right panels show the front and side views of E2, respectively. b Analysis of minimum escape time across all residues (top panel) and number of residues with relatively short escape times (bottom panel) in E2 regions that are targeted by antibodies. Among all domains accessible to antibodies, antigenic domains C and E (shaded) have (102 generations) and are thus predicted to be difficult to escape. Domain C, in particular, is predicted to be the most escape resistant. c Escape times superimposed on the crystal structure with CD81 binding sites highlighted as spheres. Specific residues (438 and 442) associated with low escape times in CD81 binding sites are also highlighted on the figure. d Escape times superimposed on the crystal structure with antigenic domains A–E highlighted as spheres. Approximate location of specific antigenic domains is shown by dotted ellipses. HVR1 and a large part of domain E is not shown as the involved residues are not resolved in the structure