FIGURE 1.
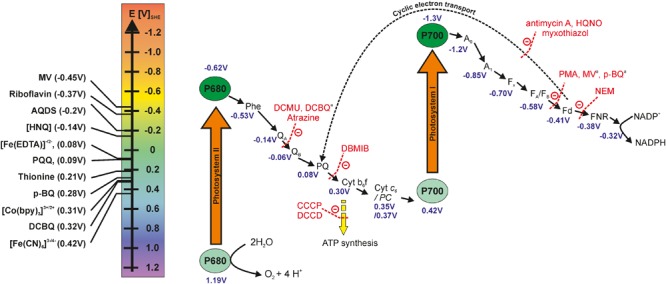
Z-scheme of the photosynthetic electron transport chain, the electron transfer inhibitors at specific sites and potential mediator molecules that could be used for withdrawing electrons. The redox potentials of photosystem I and II subunits are diverse in the literature and the values reported here are obtained from the following sources (Bottin and Lagoutte, 1992; Semenov et al., 2000; Cassan et al., 2005; Allakhverdiev et al., 2010, 2011; Kothe et al., 2013; Caffarri et al., 2014; Schuurmans et al., 2014). The redox potentials of mediators are taken for neutral aqueous conditions from Nivinskas et al. (2002),Schuurmans et al. (2014), Lai et al. (2016), and Emahi et al. (2017). AQDS, 9,10-anthraquinone-2,6-disulfonate; CCCP, carbonyl cyanide m-chlorophenylhydrazone; DCMU, 3-(3,4-Dichlorophenyl)-1,1-dimethyl urea; DCBQ, 2,6-Dichloro-1,4-benzoquinone; DBMIB, 2,5-dibromo-3-methyl-6-isopropyl-P-benzoquinone; DCCD, N-N’-dicyclohexylcarbodiimide; HNQ, 2-hydroxy-1,4-naphthoquinone; HQNO, 2-heptyl-4-hydroxyquinoline n-oxide; MV, methyl-viologen; NEM, N-ethylmaleimide; PMA, phenylmercuric acetate; p-BQ, p-benzoquinone. aDCBQ, MV and p-BQ are performing more as competitors for the natural electron acceptor rather than inhibitors that bind and block the activities of specific sites (Ravenel et al., 1994).
